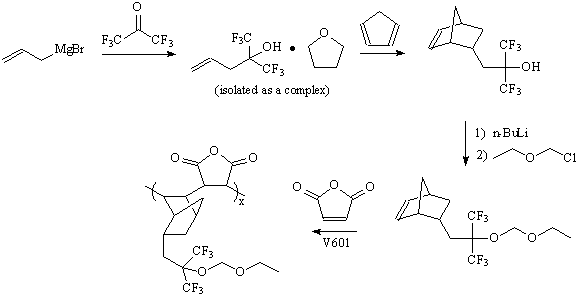
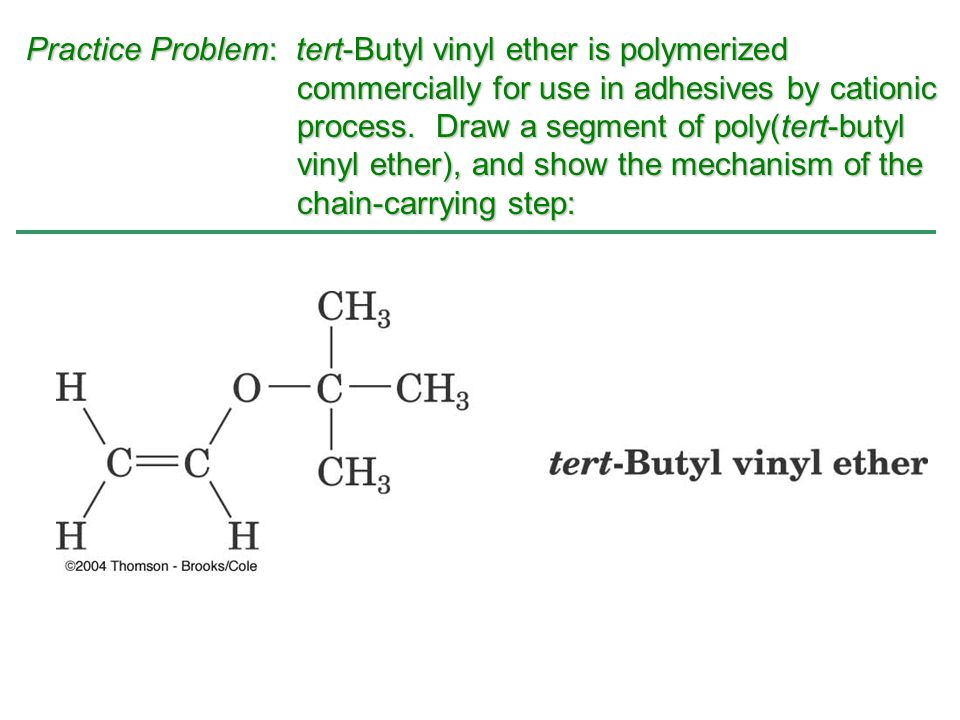
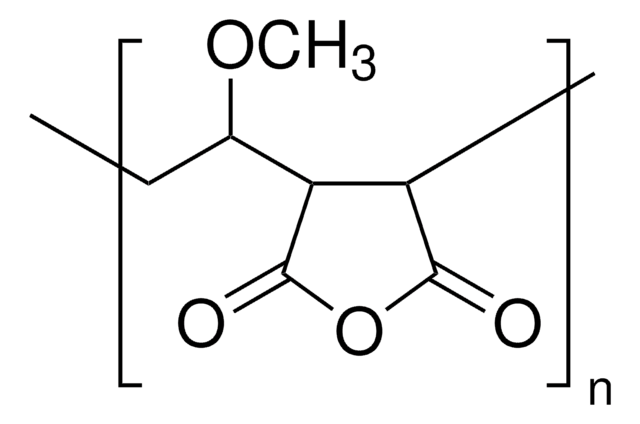
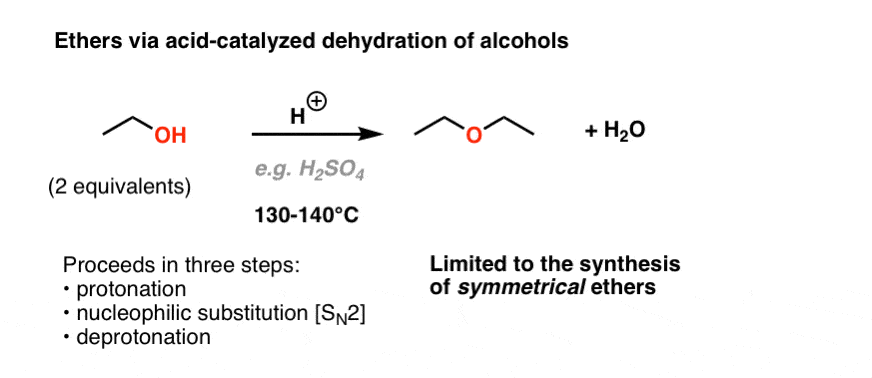
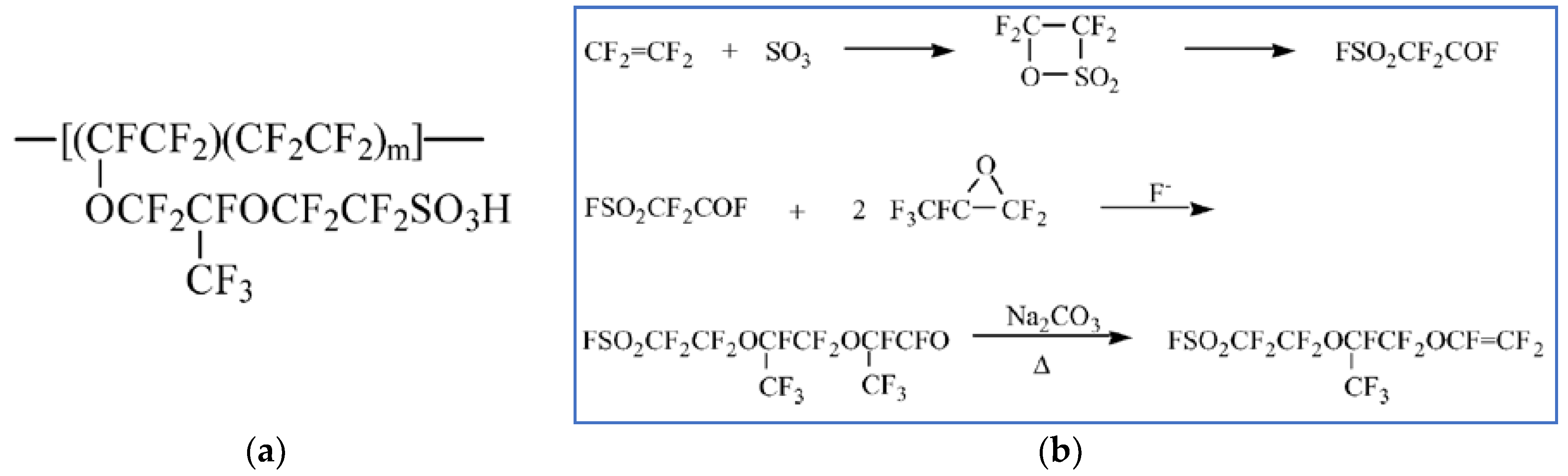
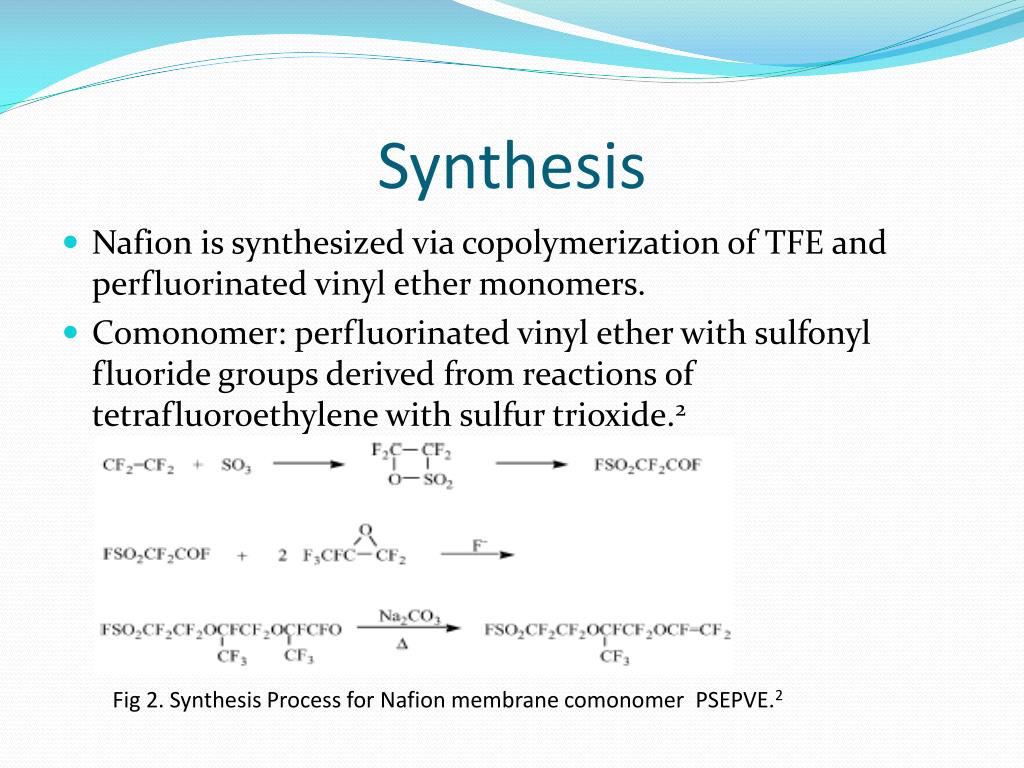
Vinyl Ether Process

The half life for this reaction in air is estimated to be 6 5 days 4 src.
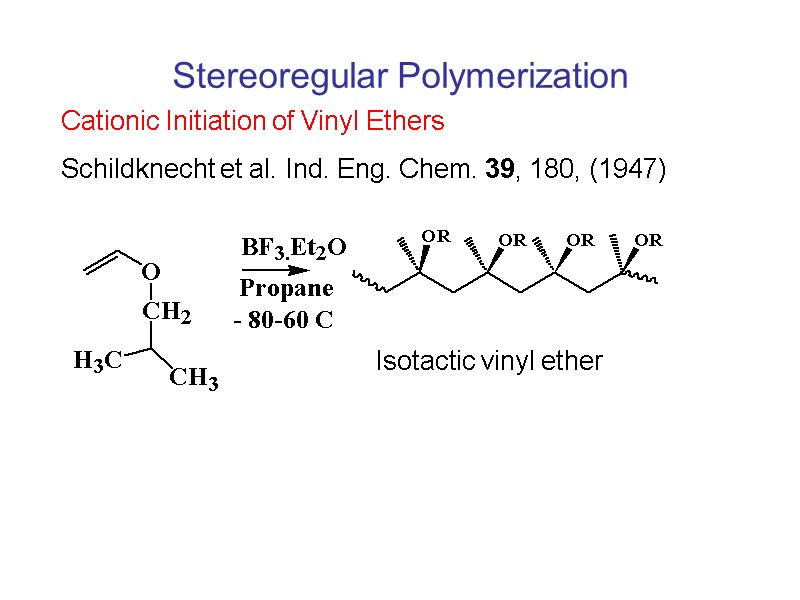
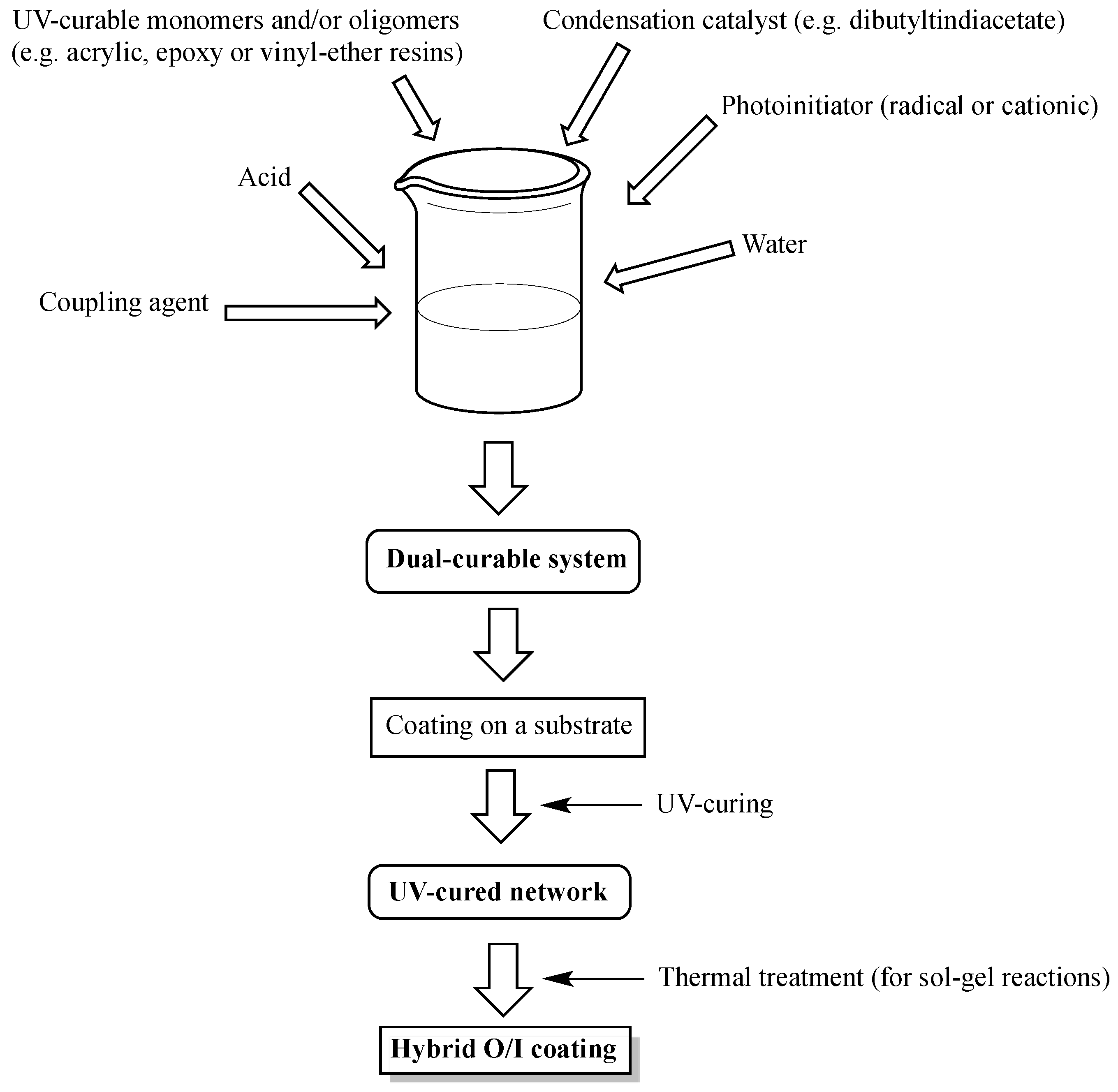
Vinyl ether process. The use of epoxide vinyl ether combinations alleviates this problem and the presence of the vinyl ether increases the cure speed compared to a formulation in which the vinyl ether is absent 21. They are increasingly used in radiation curing systems because of a lower toxicity profile than the commonly used acrylic monomers. Both polymers exhibited t g s 88 and 61 c respectively well above the room temperature. The ratio of the anesthetic to lethal does for vinyl ether is 1 to 2 4 ethyl ether.
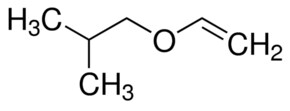
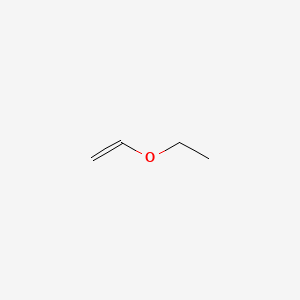
Ethyl vinyl ether is an organic compound with the chemical formula ch 3 ch 2 och ch 2. A process for efficiently producing a high purity vinyl ether represented by formula 1 from a tertiary alcohol having low reactivity is provided the process comprising reacting acetylene with the tertiary alcohol in the presence of a base using a cyclic urea compound a glyme compound or a mixture of these as a solvent. It is used as a synthetic building block and a monomer. However this potency is hard to control with simplistic equipment.
Butyl vinyl ether may be susceptible to photooxidation via vapor phase reaction with ozone. Vinyl ethers undergo radical initiated copolymerization in the presence of specific monomers such as maleates fumarates and acrylics. Many of the vinyl ethers exhibit good solvating properties and have been used as reactive diluents fo this purpose. Poly methyl vinyl ether alt maleic acid monoethyl ester solution1 product result match criteria.
Vinyl ether process ch polymer reacting prior art date 1998 09 28 legal status the legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed expired fee related application number us09 161 889 inventor lee landis blyler jr. The process involved capping the initiator tmpcl with dte in the presence of ticl 4 followed by fine tuning of the lewis acidity with the addition of ti oip 4 to match the reactivity of tert butyl vinyl ether or chve. Vinyl ether is a potent anesthetic giving it a large safety margin.
According to a classification scheme 7 an estimated bcf of 3 src from an estimated log kow of 0 42 2 and a regression. Vinyl methyl ether is expected to undergo hydrolysis in the environment based on hydrolysis half lives of 9 5 hours 40 days and 10 9 years at ph 5 6 and 9 respectively in structurally similar butyl vinyl ether 6. It is the simplest enol ether that is liquid at room temperature.