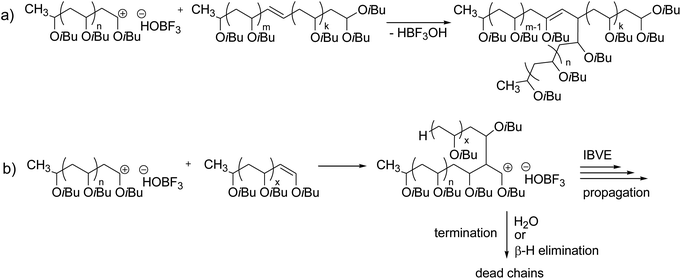
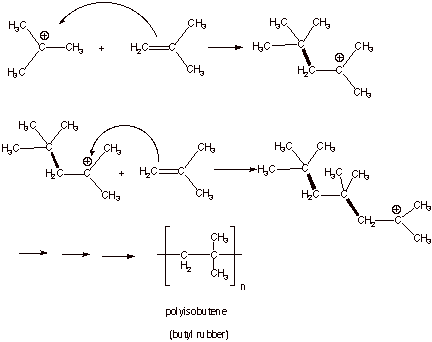
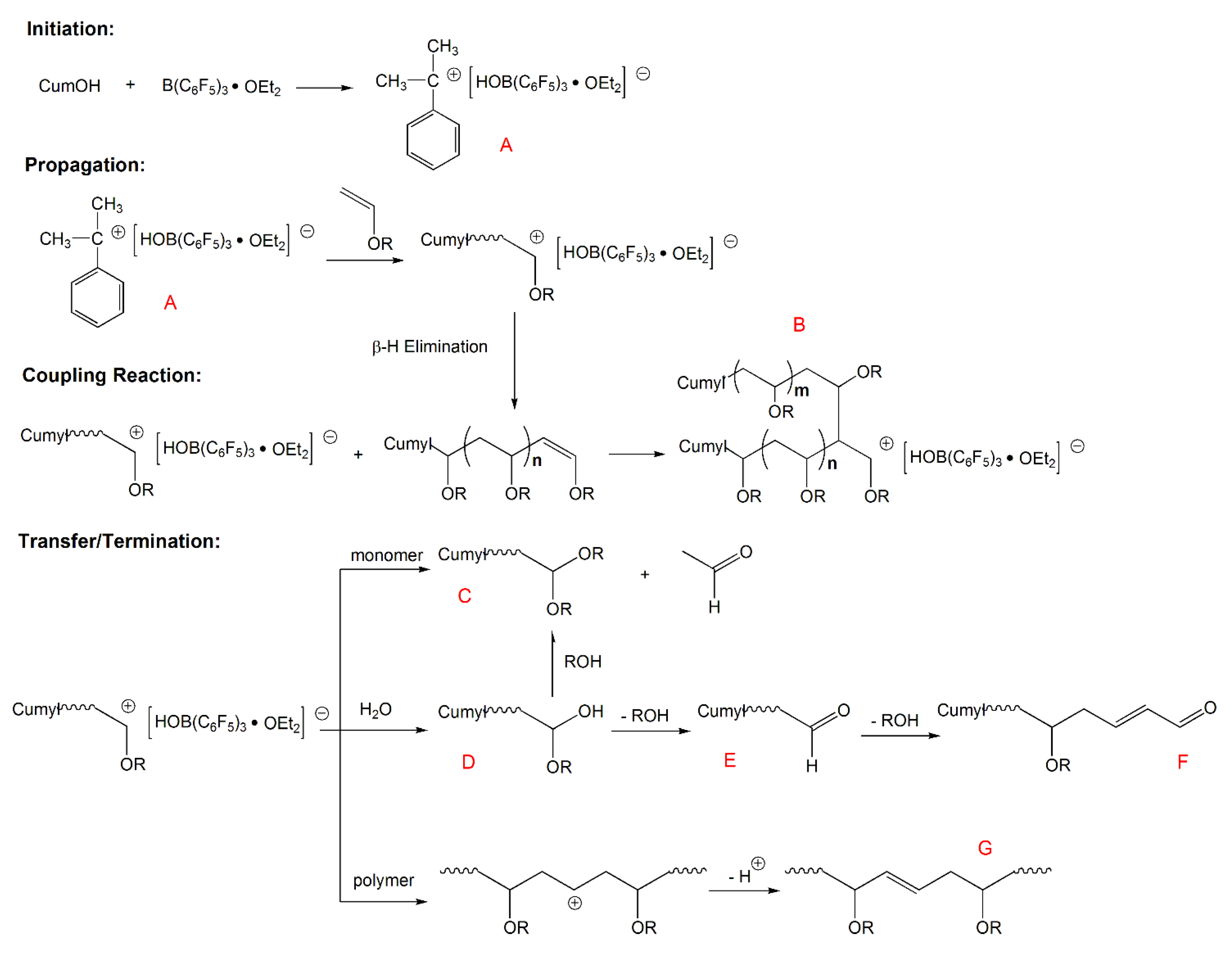
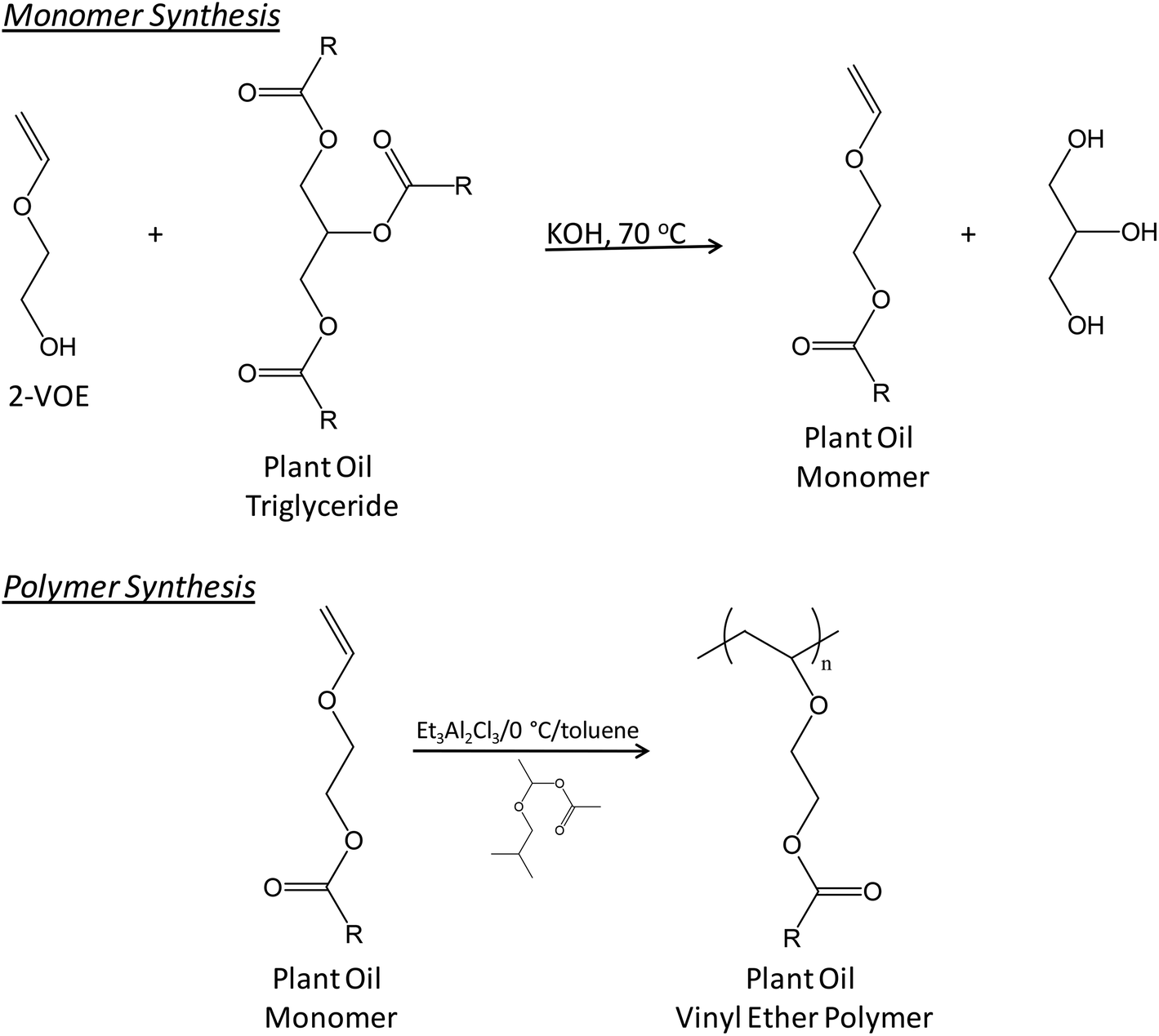
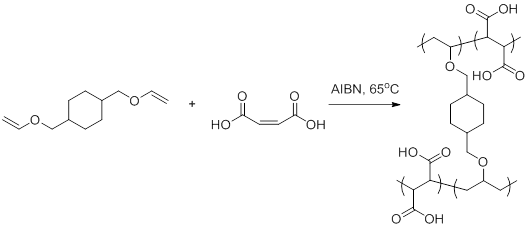
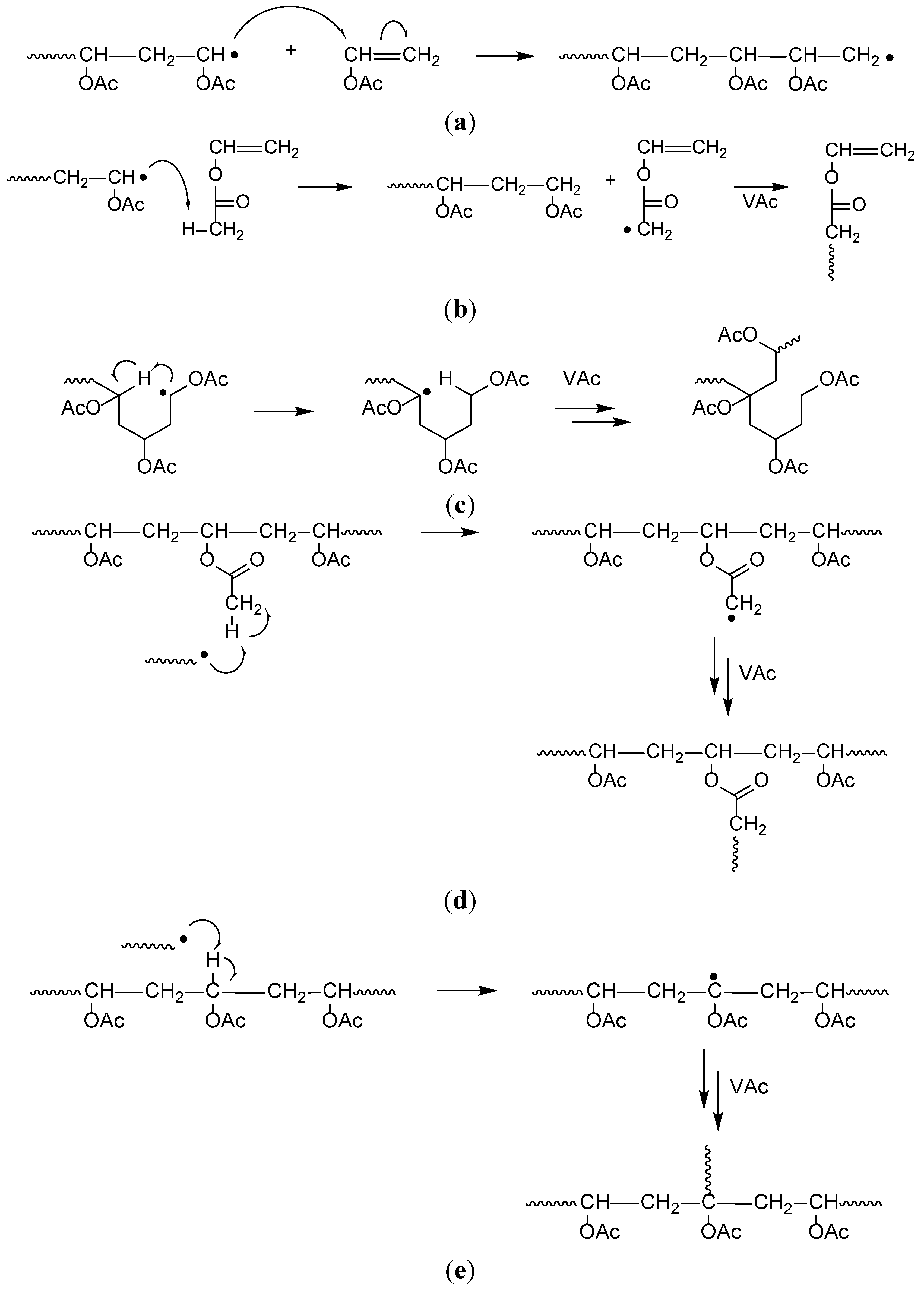
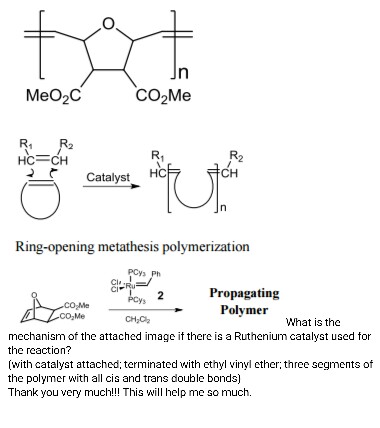
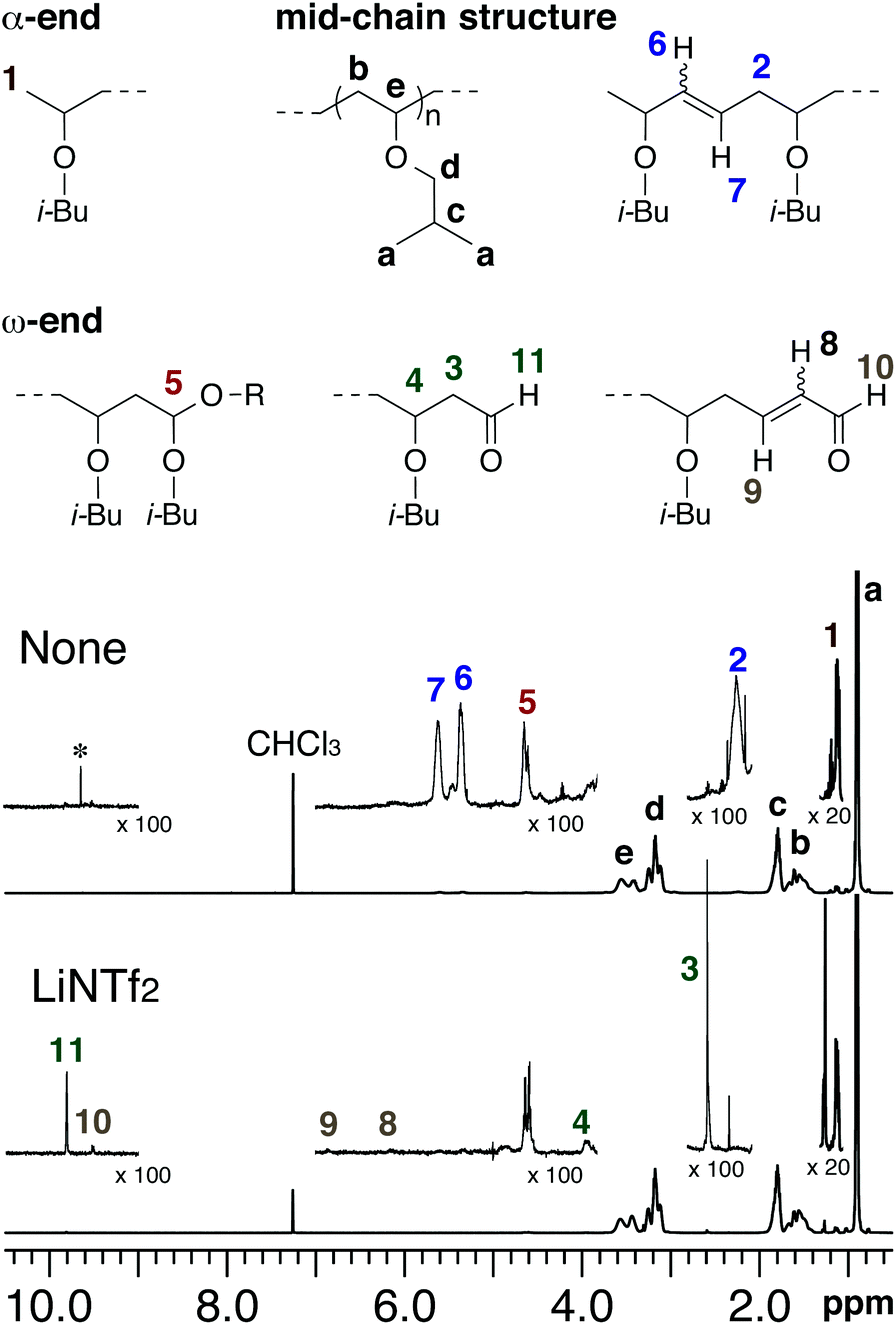
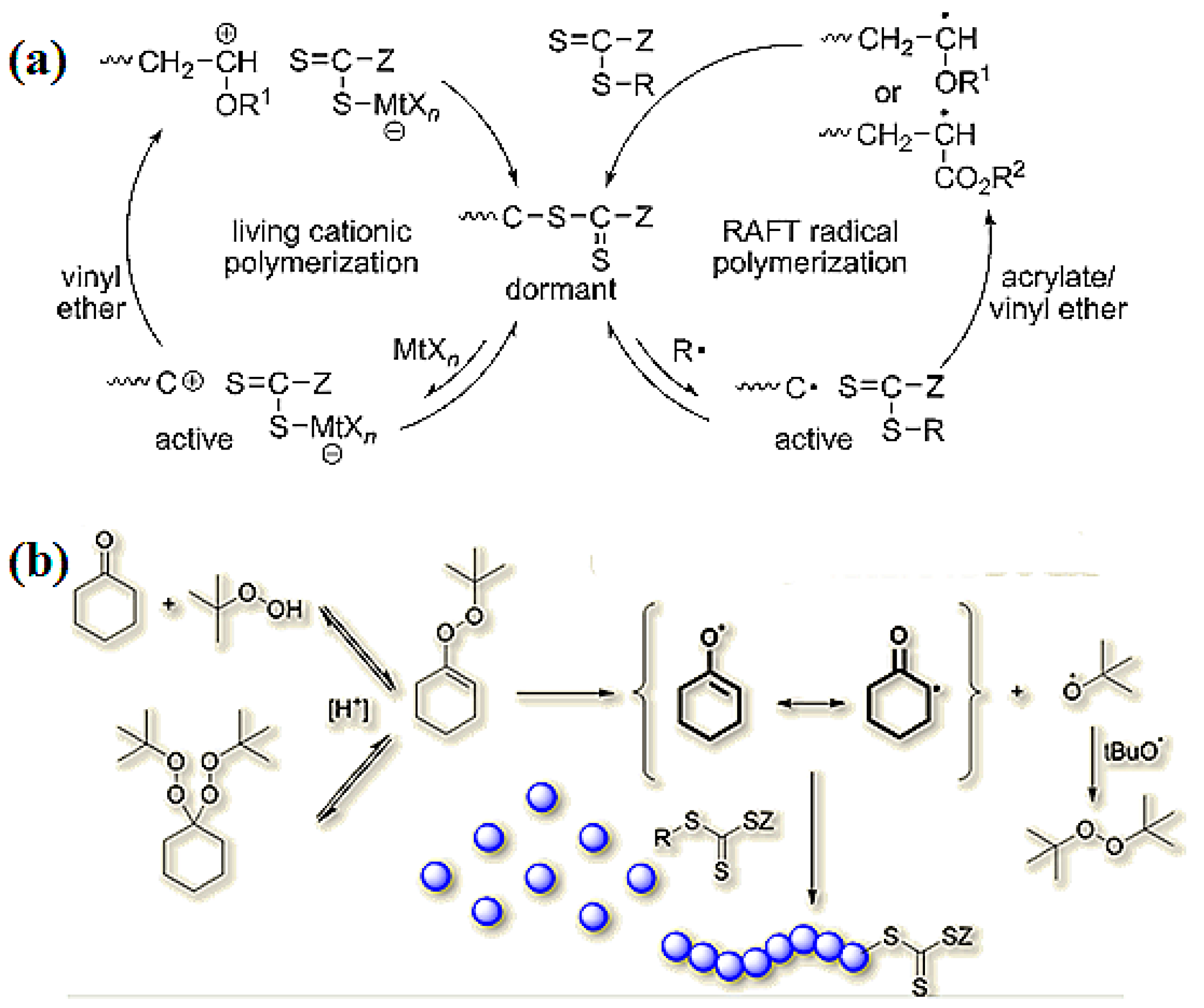
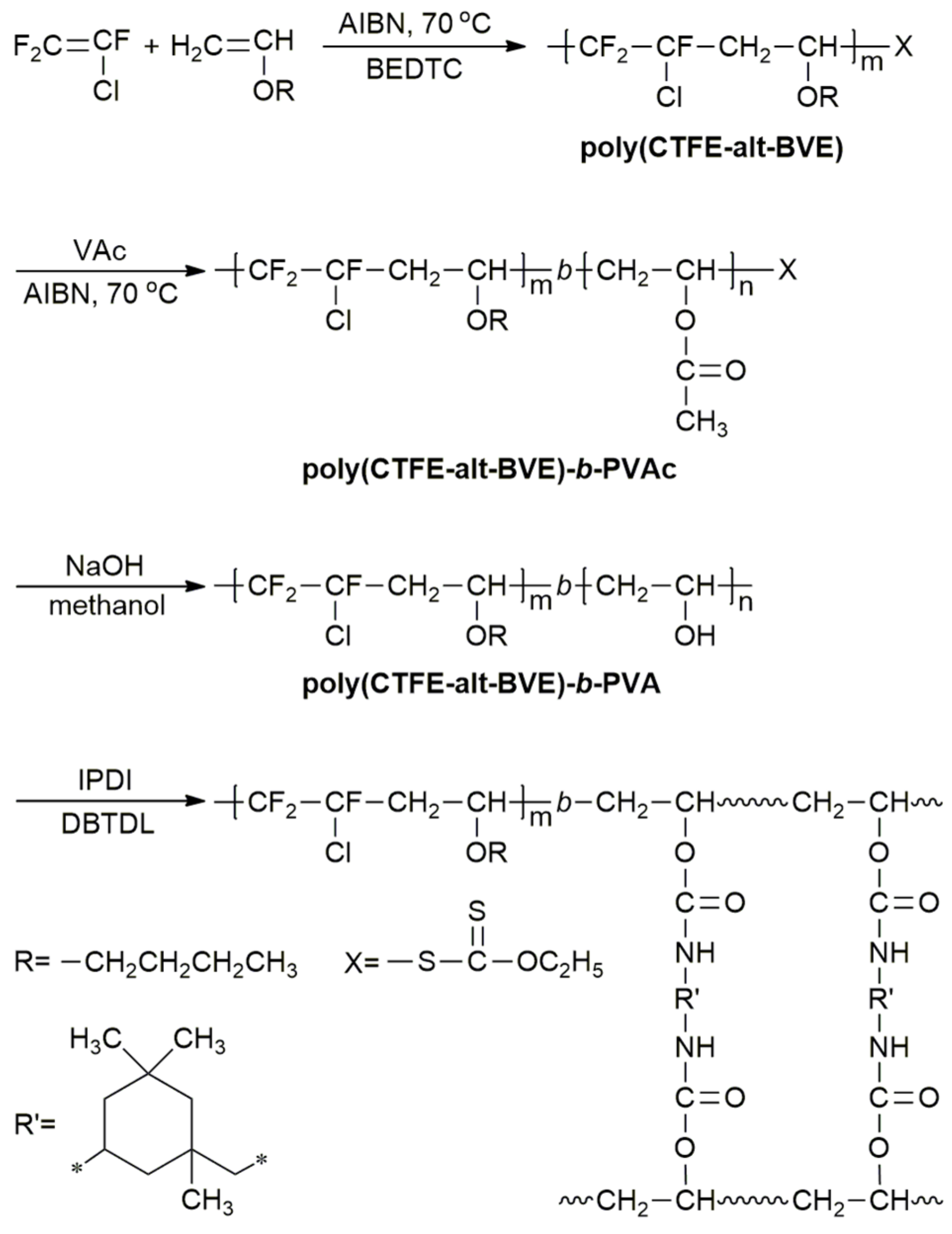
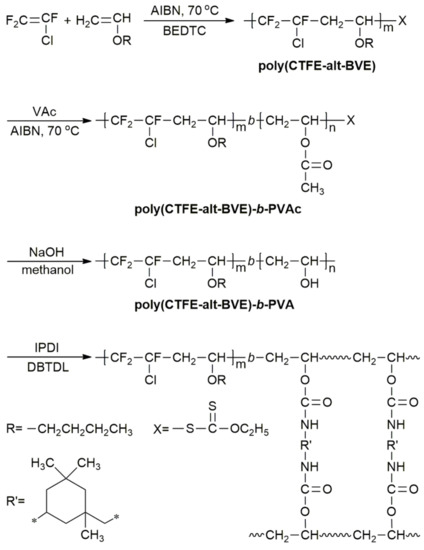
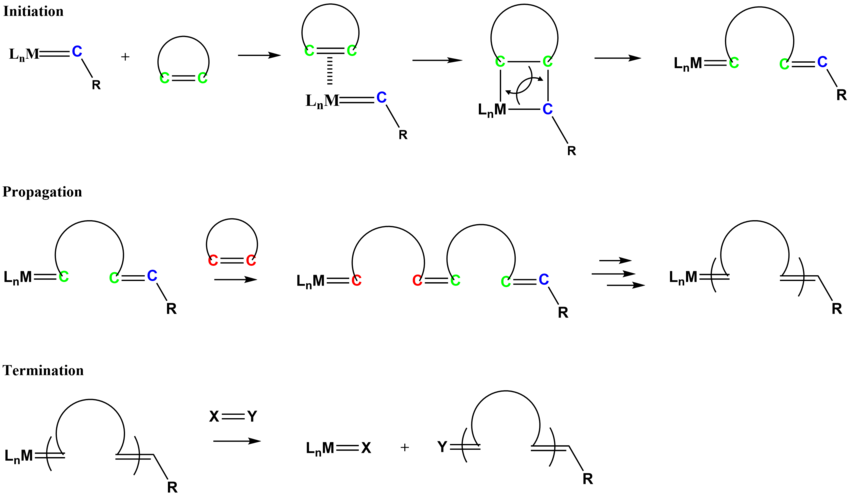
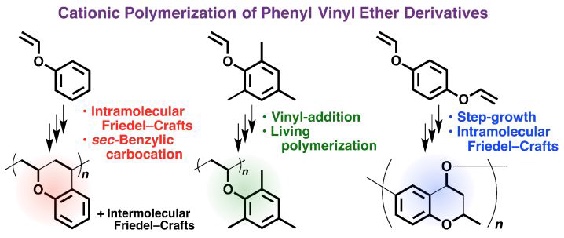
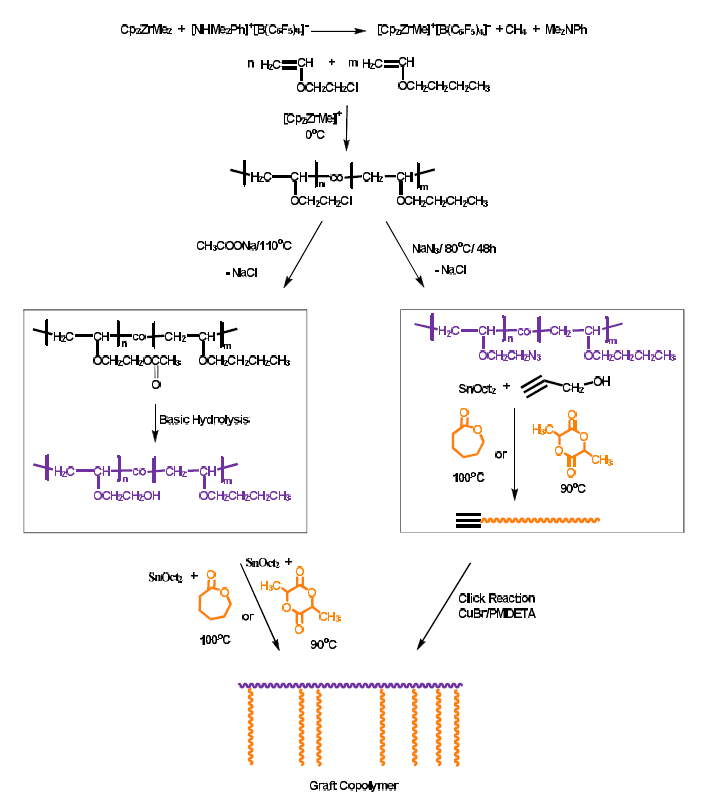
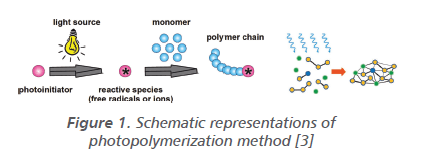
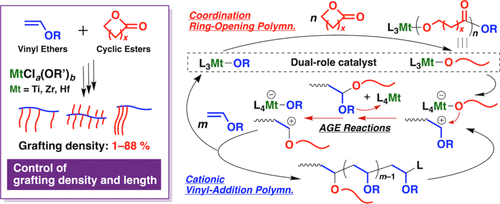
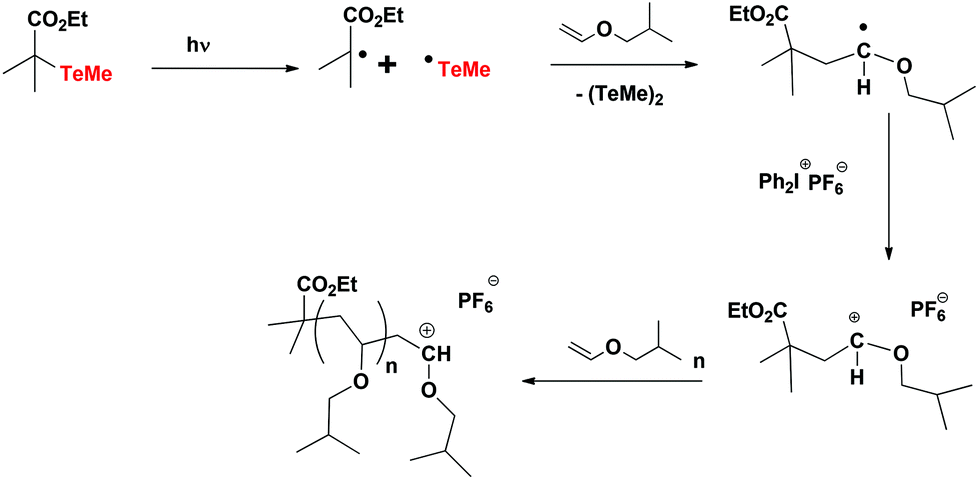
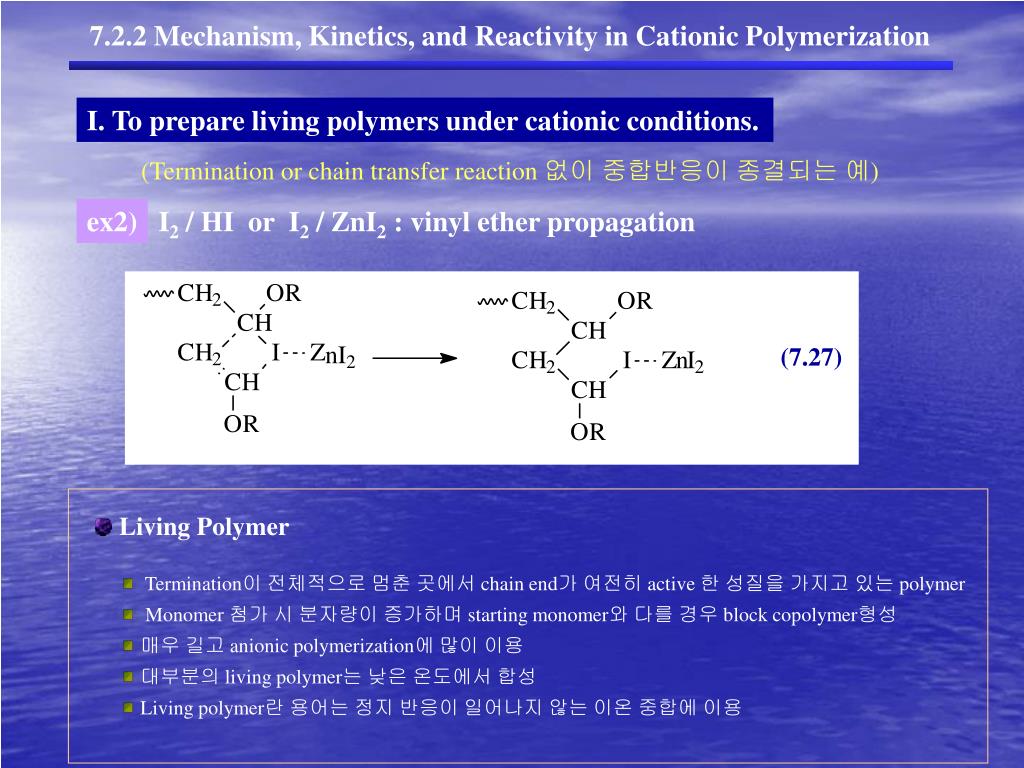
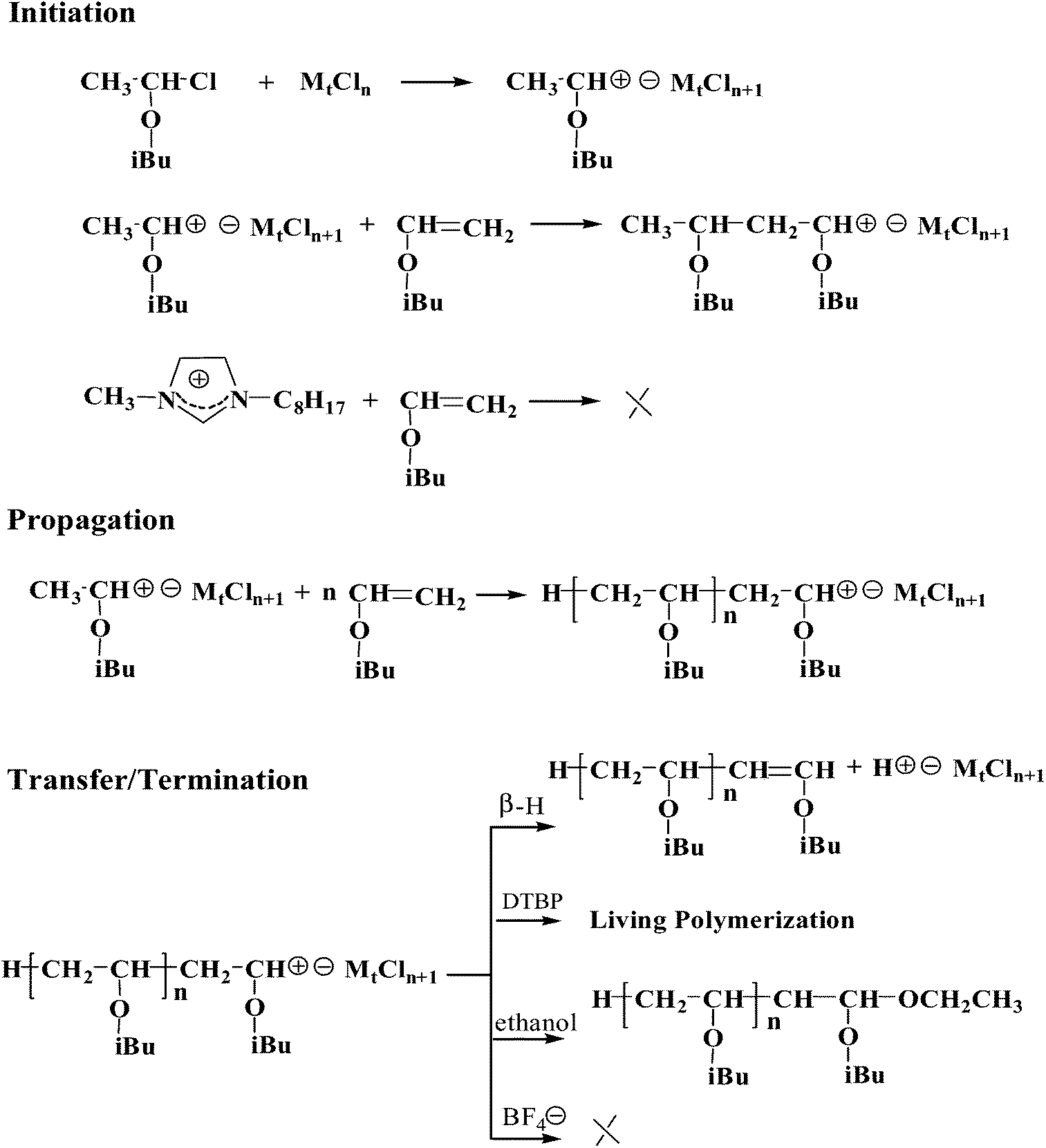Vinyl Ether Polymerization Mechanism
124 the fast living cationic polymerization of vinyl ethers with sncl 4 combined with etalcl 2 in the presence of an ester as an.
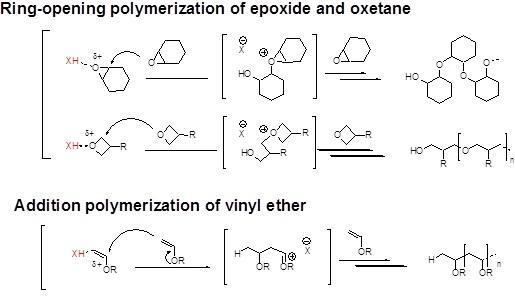
Vinyl ether polymerization mechanism. When highly reactive cycloaliphatic epoxides are subjected to photoinitiated cationic polymerization in the presence of vinyl ethers the two polymerizations proceed in a sequential fashion with the vinyl ether. Poly vinyl ether s with a t g as high as 100 c have been obtained in the living cationic polymerization of vinyl ethers with a bulky tricyclodecane or tricyclodecene unit using hcl zncl 2 in toluene at 30 c. Design of benign initiator for living cationic polymerization of vinyl ethers. This oxidation is followed by mesolytic cleavage.
Furthermore they are highly attractive monomers for the synthesis of many polymers and copolymers. Vinyl ethers ch 2 chor r methyl ethyl isobutyl benzyl are very reactive vinyl monomers. The polymerization proceeded in a reproducible manner through the careful design of experimental conditions adding initiator co solvents and. However it is challenging to incorporate these substrates in transition metal catalyzed olefin polymerization due to various side reactions such as cationic.
Kanazawa a hashizume r kanaoka s. Polymerization is typically initiated with lewis acids such as boron trifluoride. For example the state of the art method uses a phenoxide ligated titanium complex to achieve 92 meso diads m in the polymerization of iso butyl vinyl ether ibve. Methyl vinyl ether can be made by reaction of acetylene and methanol in presence of a base.
The electron rich nature of vinyl ethers ch2 chor enables their versatile reactivity patterns with transition metal catalysts. It is prone to polymerization leading to formation of polyvinyl ethers. Living cationic ring opening polymerization. Studied systems are based on i 2 hi and on zinc halides zinc chloride zinc bromide and zinc iodide.
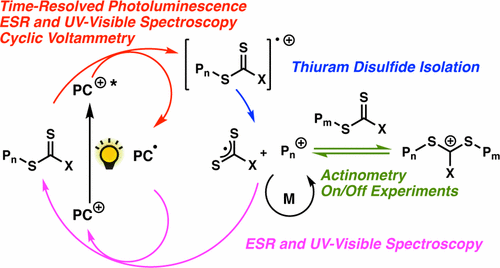
Our study revealed a complex activation step characterized by one electron oxidation of the cta. The mechanism of the recently reported photocontrolled cationic polymerization of vinyl ethers was investigated using a variety of catalysts and chain transfer agents ctas as well as diverse spectroscopic and electrochemical analytical techniques. Aqueous cationic polymerizations of vinyl ethers isobutyl vinyl ether ibve 2 chloroethyl vinyl ether ceve and n butyl vinyl ether n bve were performed for the first time by a cumoh b c6f5 3 et2o initiating system in an air atmosphere. In all cases the rate of epoxide ring opening polymerization is accelerated whereas that of the vinyl ether is depressed.
The alkene portion of the molecule is reactive in many ways. The isotacticity of the polymer was independent of the catalyst concentration but increased with a decrease in the initial monomer.