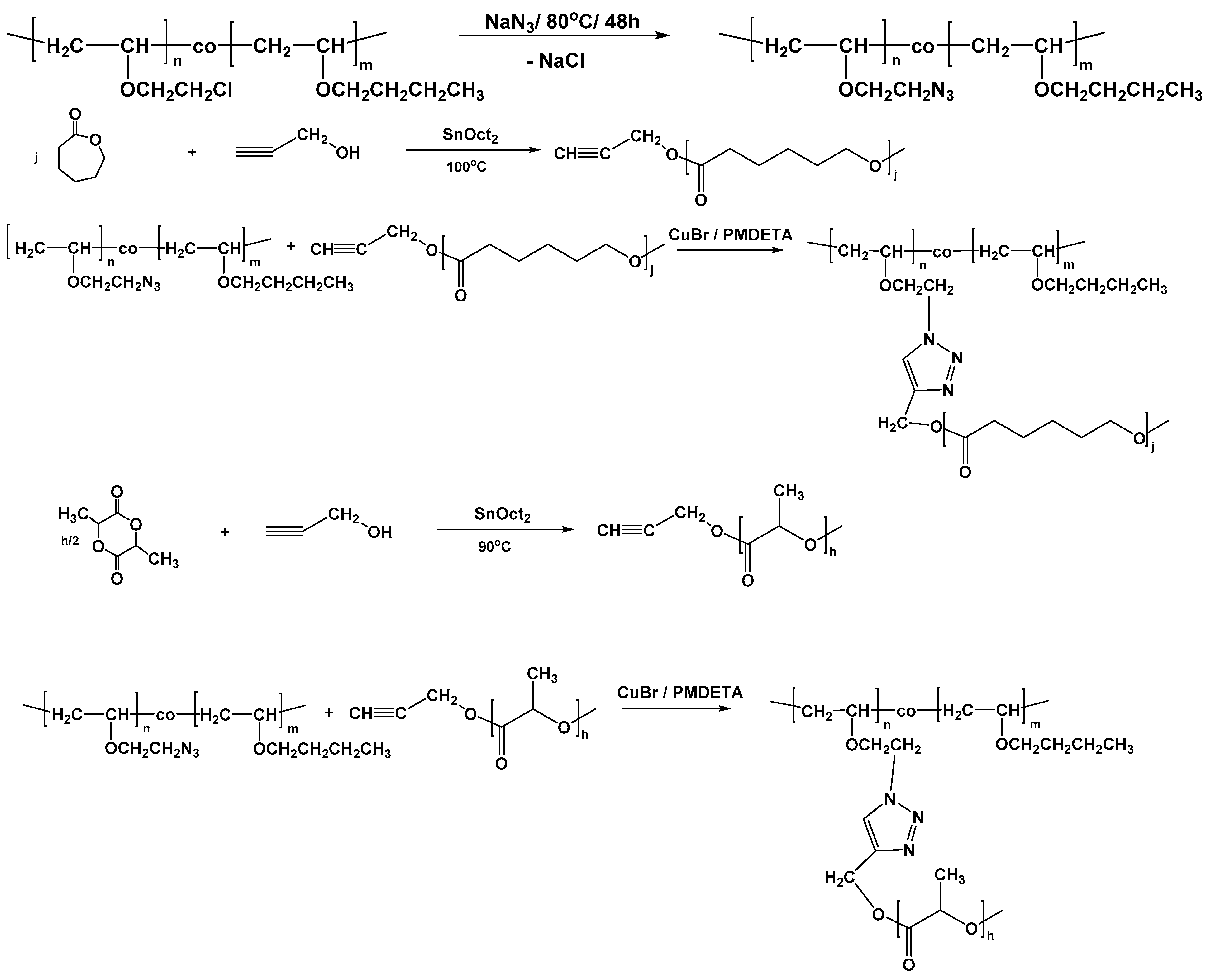
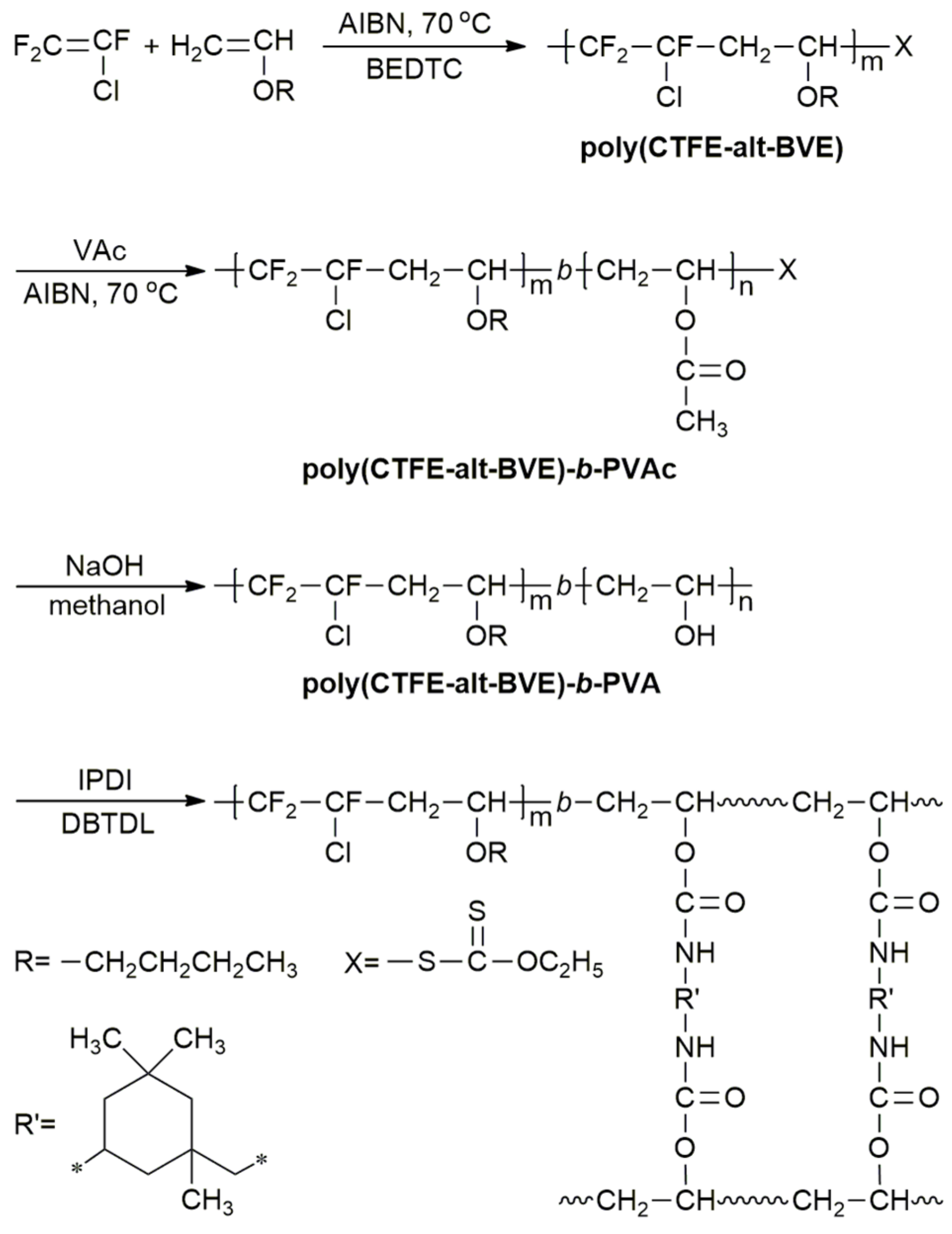
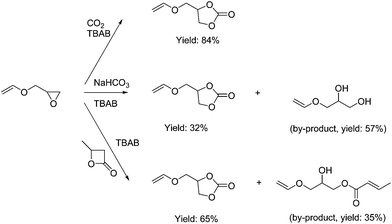
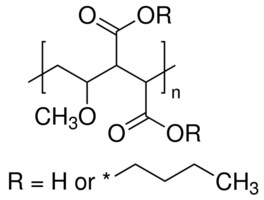
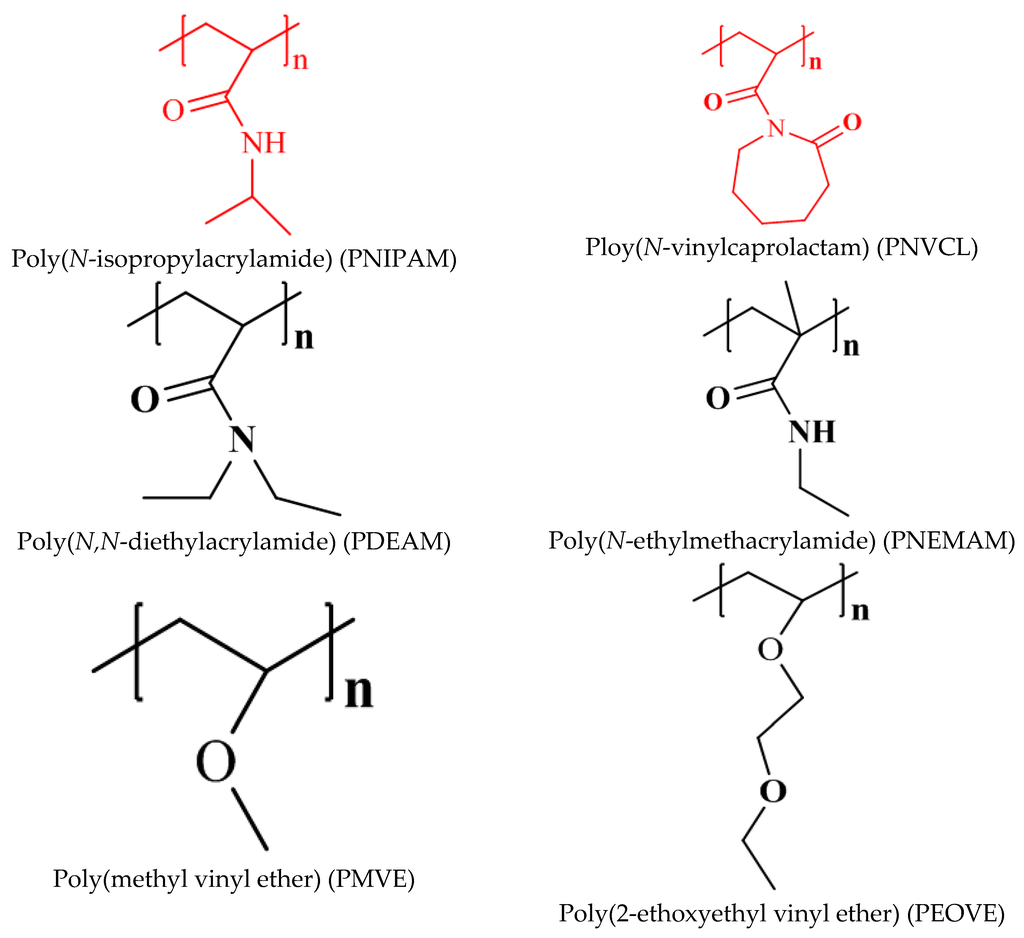
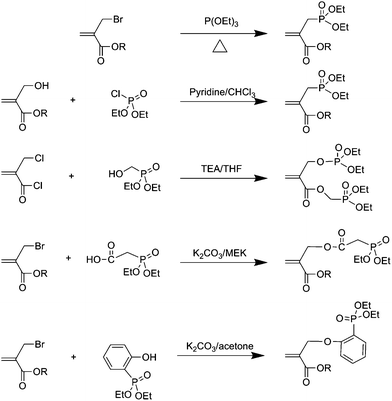
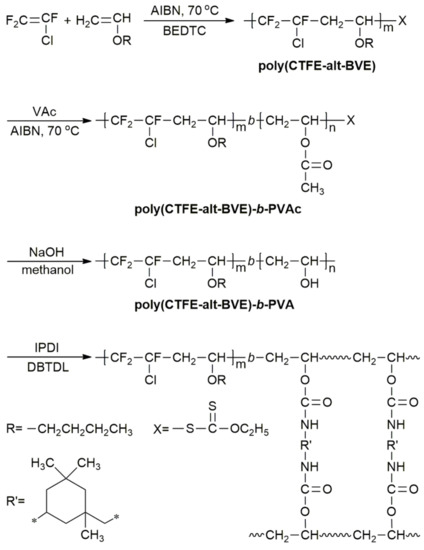
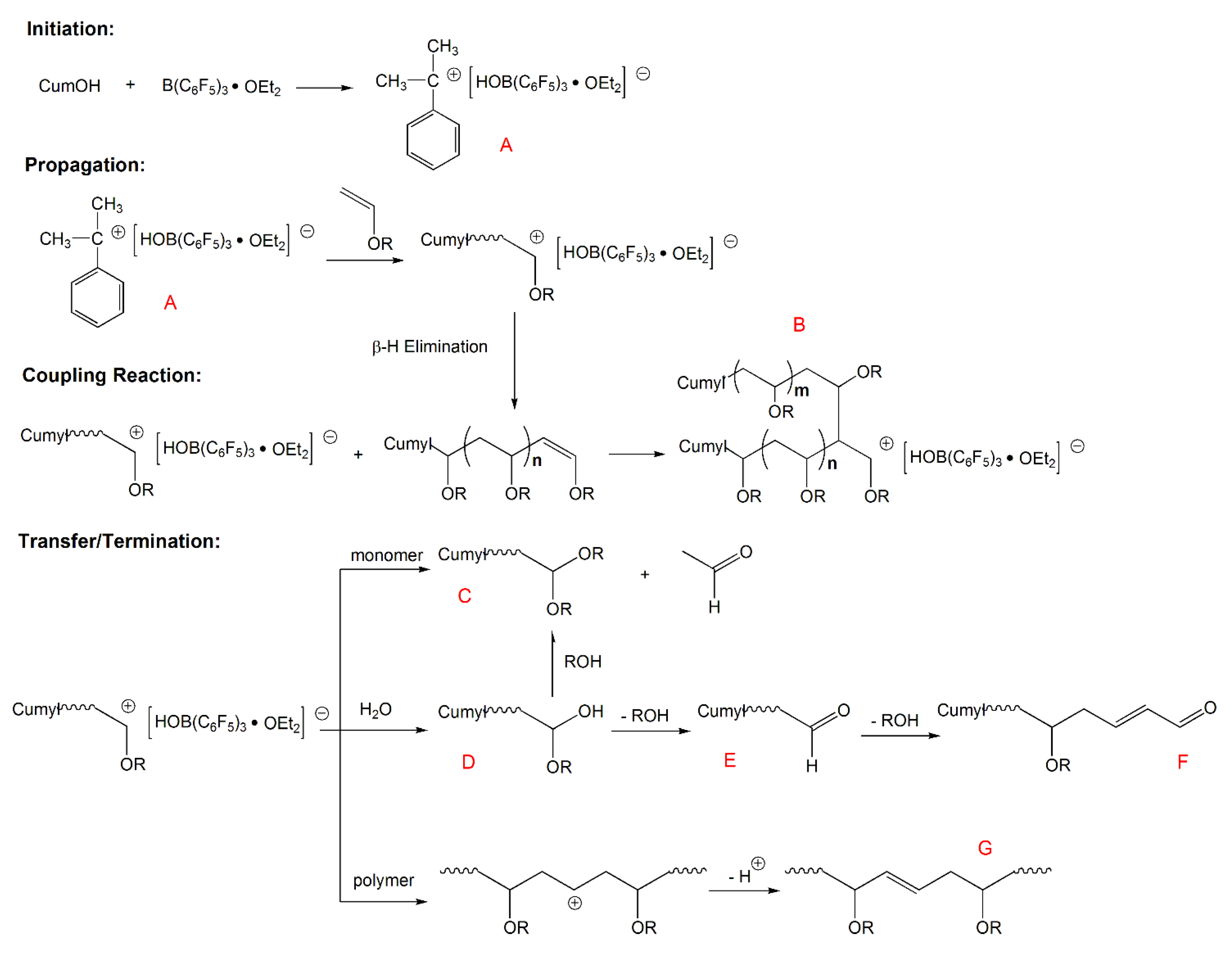
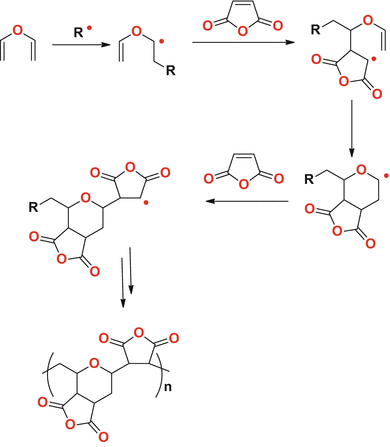
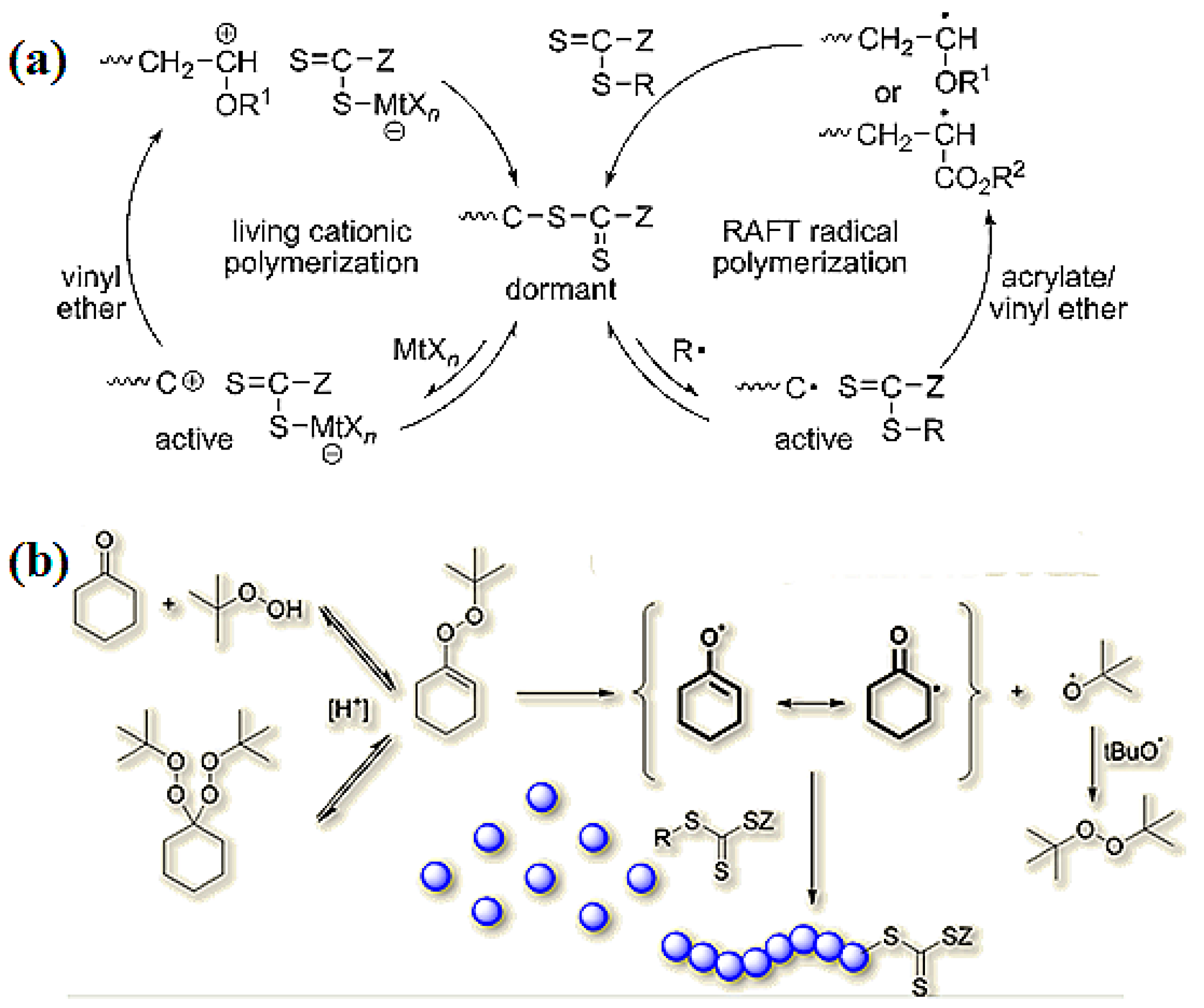
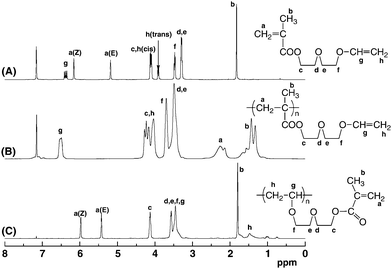
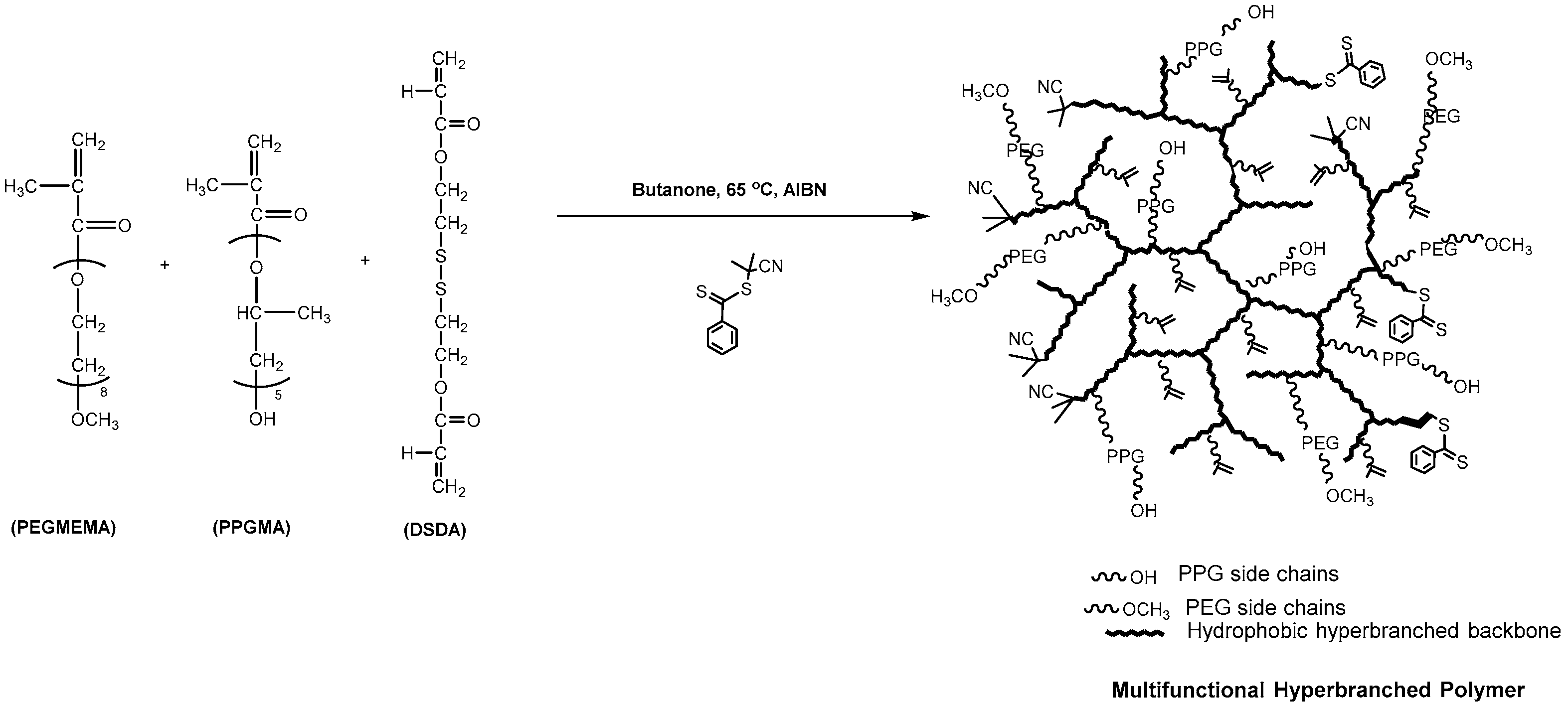
Vinyl Ether Acrylate Synthesis

They are increasingly used in radiation curing systems because of a lower toxicity profile than the commonly used acrylic monomers.
Vinyl ether acrylate synthesis. Acrylate vinyl ether radical figure 5. The acrylate modified vinyl chloride and vinyl isobutyl ether copolymers were characterized by fourier transform infrared spectroscopy. Palladium ii catalyzes the oxidative coupling of allyl tosylamides with butyl vinyl ether and various styrene derivatives to produce 2 4 substituted pyrrolidine products at room temperature. Herein we design and synthesis vinyl ether terminated cyanobiphenyl and cyanoterphenyl monomers difunctional vinyl ether liquid crystal c6v as components of v lcs mixture as depicted in scheme s1 and the details are described in the supporting information a scheme proposing the fabrication of qds films with fluorescence enhancement and encapsulation of qds was given in fig.
Molecular oxygen together with a copper ii cocatalyst mediates reoxidation of the palladium catalyst. Chemical synthesis 49 materials science 411 molecular biology 26 research essentials 84 solvents 8. Ethylene glycol methyl ether acrylate. Snider in comprehensive organic synthesis 1991.
The reactions with styrene substrates can be performed in an open flask with ambient air as the. 3 product results match criteria. The mean molecular weight of grafted polymer was determined by gel permeation chromatography and the particle sizes and their distributions of the dispersions were measured by laser light scattering. Methyl vinyl ether solution.
8 21 227 228 the mechanism for chain transfer is shown in scheme 9 for the case of α benzyloxystyrene 15 the driving force for fragmentation is provided by formation of a strong carbonyl double bond. Section 1 1 2 1 2 ii α β unsaturated. The purpose of this work is to identify special features in the properties of alkyl meth acrylate vinyl butyl ether copolymers for a number of alkyl meth acrylates in the presence of a triethylborane oxygen initiator. Vinyl ethers undergo homopolymerization via a cationic mechanism.
It is also important that r is a good radical leaving group. Section 1 1 2 1 2 i acrylate esters. Narasaka and coworkers found that n acryloyloxazolidinone undergoes a 2 2 cycloaddition with 1 1 bis methylthio ethylene in the presence of ticl 2 opr i 2 and 213 to give 74 of the cyclobutane in 88 enantiomeric excess. Polymerization rate in figure 5 the conversion of acrylate 1407 cm 1 and vinyl ether groups 1618 cm 1 are plotted as a function of vinyl ether content.
Vinyl ethers 1 x ch 2 a o can be very effective addition fragmentation chain transfer agents. Product name description synonym.