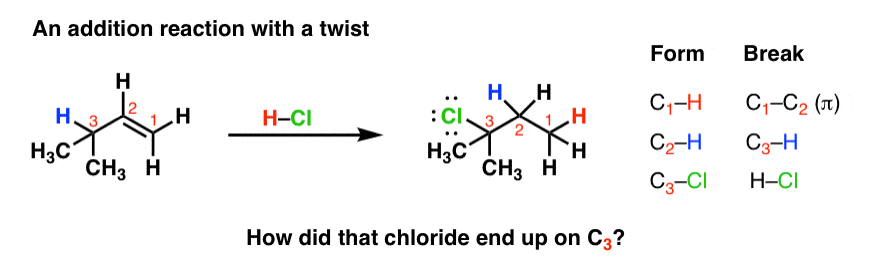
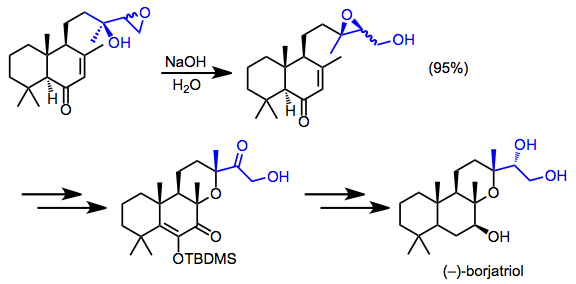
Vinyl Epoxide Rearrangement

Paréc a département de chimie et biochimie université de moncton moncton nb e1a 3ea canada b instituto de quimica unam 04510 mexico df mexico.
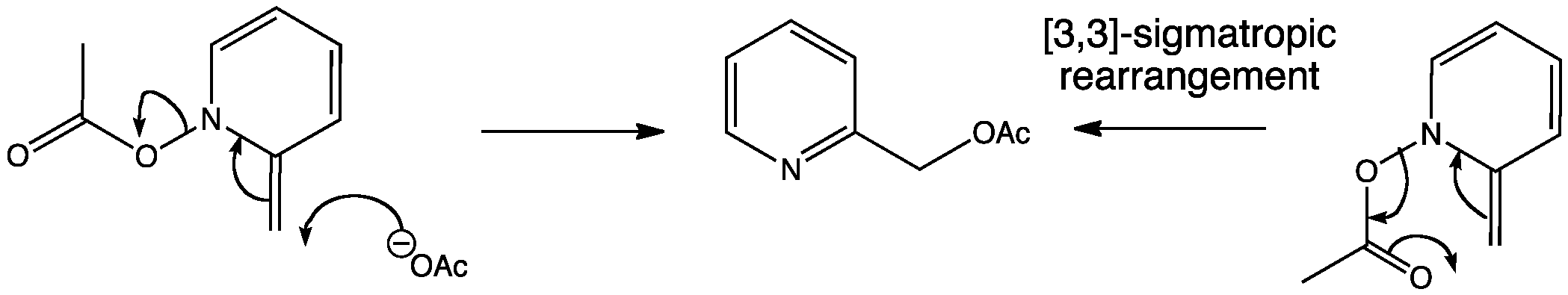
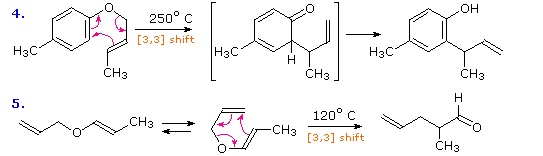
Vinyl epoxide rearrangement. Chao hu ren jie song ming hu yuan yang jin heng li shenglian luo. The aliphatic claisen rearrangement is a 3 3 sigmatropic rearrangement in which an allyl vinyl ether is converted thermally to an unsaturated carbonyl compound. This method uses low catalyst loadings 0 5 5. Spectroscopy 19 2005 171 180 171 ios press brief communication on the mechanism of a new dihalocyclopropane dihalomethyl vinyl rearrangement c k.
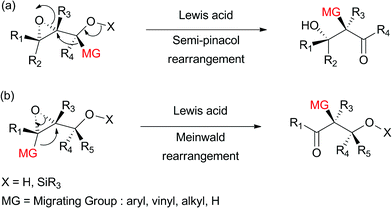
The practical stereocontrolled synthesis of vicinal halohydrins and haloamines from vinyl epoxides and vinyl aziridines. The rearrangement of acyl nitrenes to isocyanates that is the crux of the hofmann curtius and lossen rearrangements is paralleled by the rearrangement of acyl carbenes to ketenes a transformation called the wolff rearrangement this rearrangement is a critical step in the arndt eistert procedure for elongating a carboxylic acid. Regioisomeric vinyl oxiranes can be converted to a single dihydrofuran product using these conditions. It was originally discovered by ivan nikolaevich nazarov 1906 1957 in 1941 while studying the.
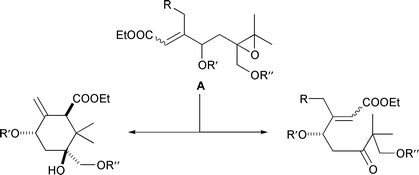
A wide range of vinyl oxiranes can be rearranged to 2 5 dihydrofurans in excellent yields in the presence of electrophilic copper ii acetylacetonate catalysts. Alewis acid catalyzed rearrangement of vinyl epoxide 11 should give rise to the b g unsaturated enone of 1 through intramolecular 1 2 hydride shift in as uprafacial fashion. The nazarov cyclization reaction often referred to as simply the nazarov cyclization is a chemical reaction used in organic chemistry for the synthesis of cyclopentenones the reaction is typically divided into classical and modern variants depending on the reagents and substrates employed. 353 354 an oxonium ylide is produced on the attack by the metal carbenoid obtained from the decomposition of a diazo compound equation 94.
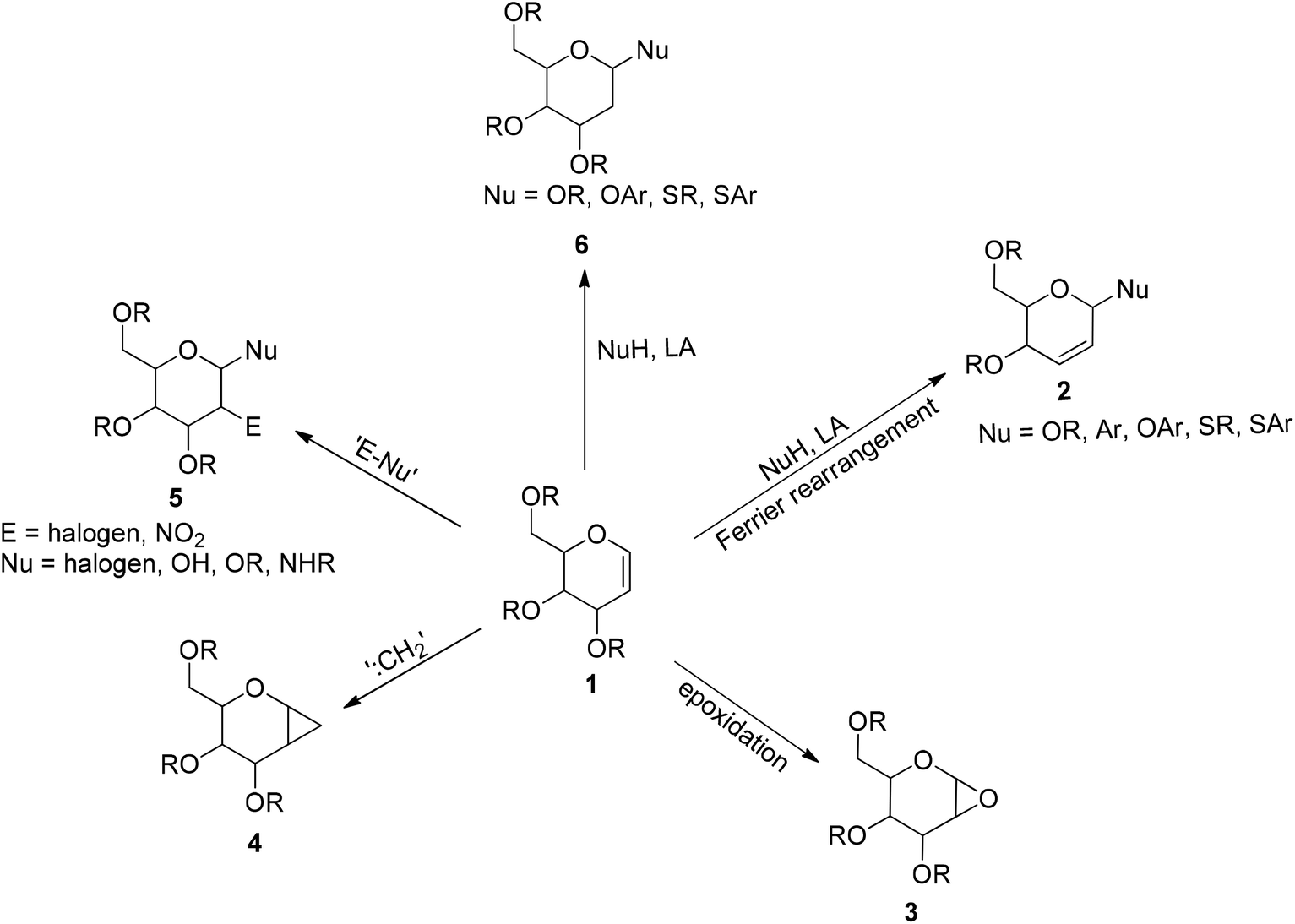
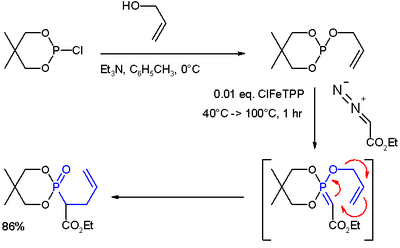
Electron poor aziridines undergo the reverse rearrangement in the presence of hydride base 13 while the corresponding epoxy amines undergo the forward rearrangement in. Tetrahedron letters 2016 57 40 4477 4479. Only two examples have been published on this rearrangement. Vinyl epoxides can be rearranged in a similar 2 3 fashion via oxonium ylides.
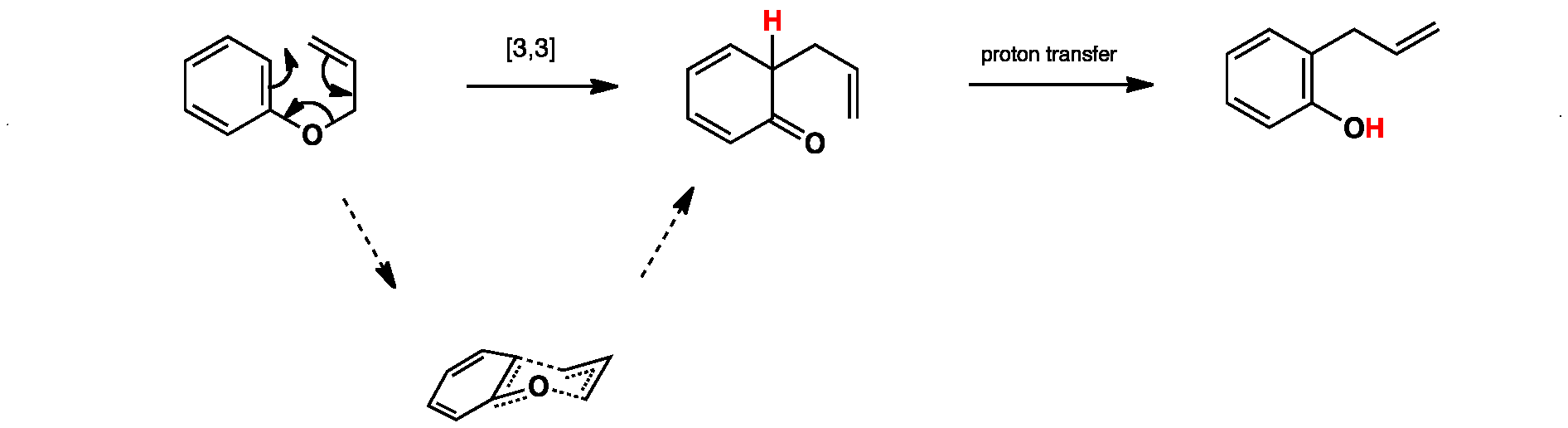
The aromatic claisen rearrangement is accompanied by a rearomatization. The reaction of epoxides with allylboranes has been reported only in the context of a concomitant meinwald rearrangement for example cyclopentadiene monoxide derivatives react with allyldialkylboranes to give acyclic trienes equation 151 most likely the reaction occurs through a zwitterionic sigmatropic reaction to give an aldehyde followed by allylation. 287 similar chemistry was.