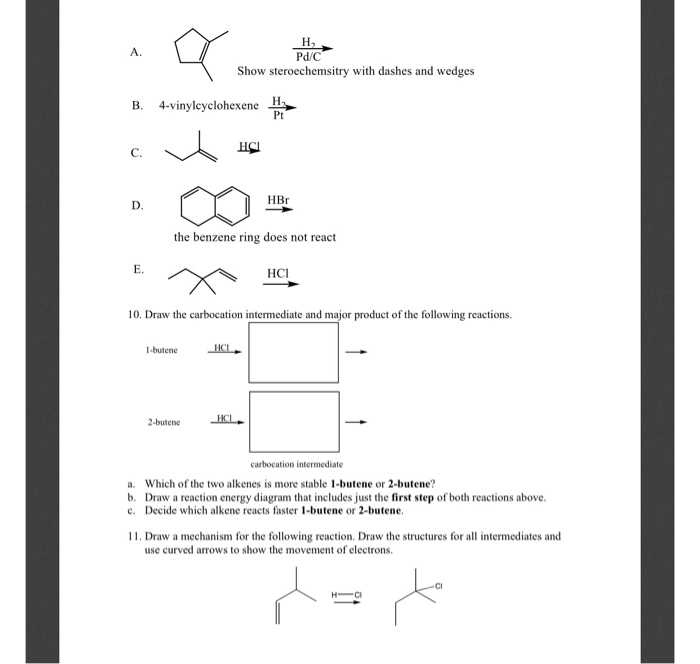
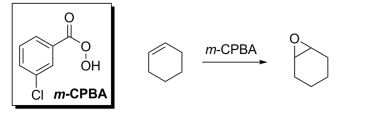
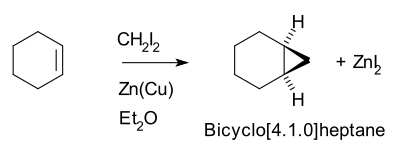
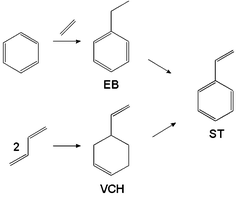
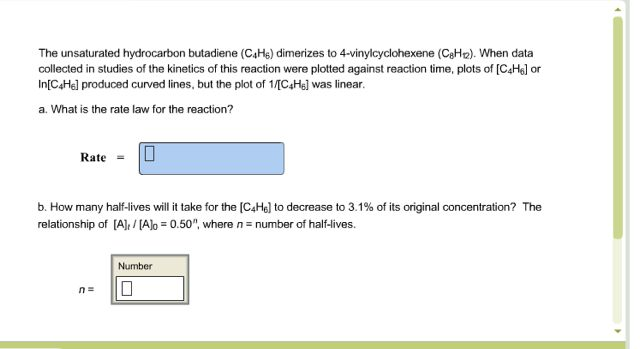
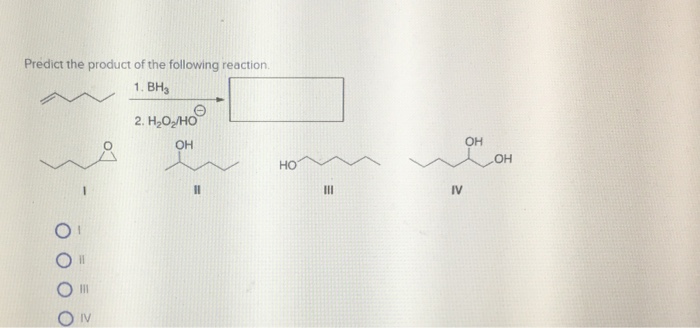
Vinyl Cyclohexene Reaction
They polymerize in the presence of catalysts or when heated.
Vinyl cyclohexene reaction. 125 at lower temperatures optically active 4 exo dideuteriovinyl cyclohexene undergoes both racemization and deuterium scrambling to c 3. Cyclohexene is produced by the partial hydrogenation of benzene a process developed by the asahi chemical company. However it slowly hydrolyzes in aqueous solutions akron 2009. The main 4 vinyl 1 cyclohexene metabolite formed in mice liver microsomes after incubation was 4 vinylcyclohexane 1 2 diol.
4 vinyl 1 cyclohexene safety data sheet print date. Conditions to avoid excess heat. Use of the information documents and data from the echa website is subject to the terms and conditions of this legal notice and subject to other binding limitations provided for under applicable law the information documents and data made available on the echa website may be reproduced distributed and or used totally or in part for non commercial purposes provided that echa is. Take off immediately all contaminated clothing.
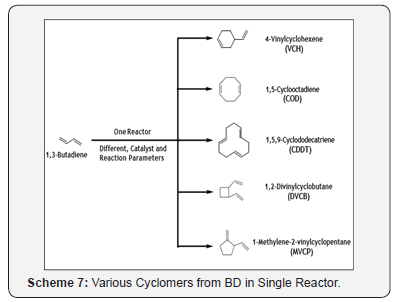
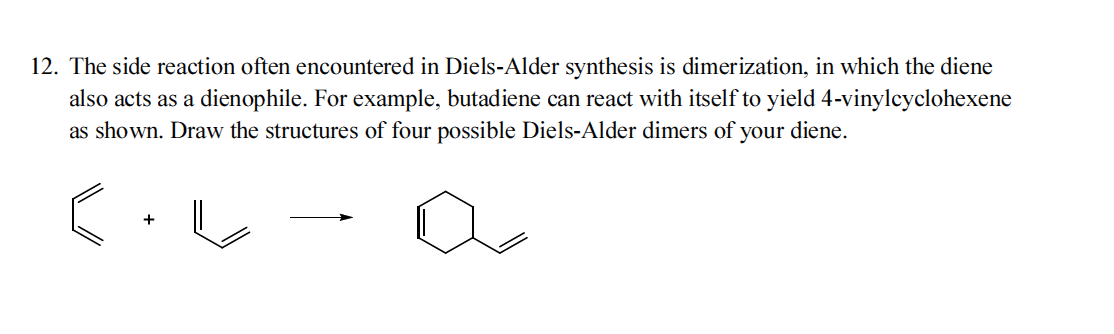
It is produced by 1 3 butadiene dimerizes in a diels alder reaction. Compounds in this group react with acids bases and oxidizing and reducing agents. Properties 4 vinyl 1 cyclohexene diepoxide is a colorless liquid at room temper ature ntp 1989. Journal of analytical and applied pyrolysis 1988 13 4 259 275.
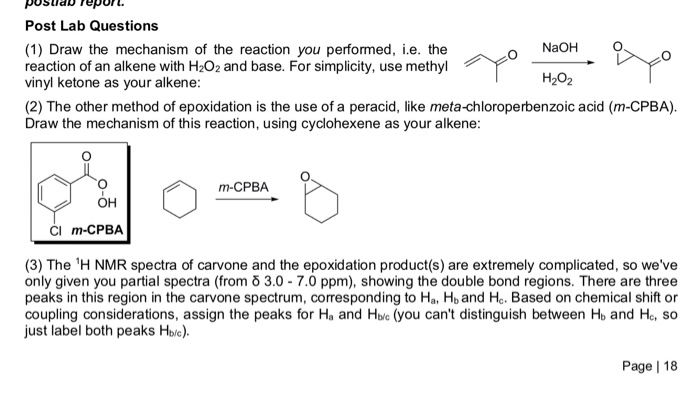
These polymerization reactions can be violent. Epoxides are highly reactive. 4 vinylcyclohexene undergoes a retro diels alder reaction to butadiene with log k 15 2 62000 2 3rt 124 or 15 7 61 800 2 3rt. It is a colorless liquid.
Physical and chemical properties of 4 vinyl 1 cyclohexene diepoxide are listed in the following table. Benzene is converted to cyclohexylbenzene by acid catalyzed alkylation with cyclohexene. 4 vinyl 1 cyclohexene stabilized revision date 18 jan 2018 decomposition temperature no information available viscosity 0 7 mpa s at 20 c molecular formula c8 h12 molecular weight 108 18 10. Other metabolites were 4 vinyl 1 2 epoxycyclohexane 4 vinyl 1 cyclohexene dioxide.
4 vinyl 1 cyclohexene dioxide increased approx 10 times the forward mutation rate of v79 chinese hamster cells. It is soluble in water. Rearrangement reactions in the thermal formation of aromatics from cycloolefins. 1 vinyl 3 cyclohexene dioxide reacts with active hydrogen compounds such as alcohols and amines.
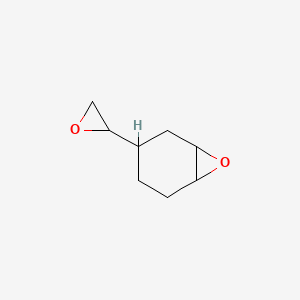
It is a precursor to vinylcyclohexene dioxide. Stability and reactivity reactive hazard none known based on information available stability stable under normal conditions. 4 vinylcyclohexene is an organic compound consisting of a vinyl group attached to the 4 position of the cyclohexene ring. Although chiral it is used mainly as the racemate.
126 these processes occur with log k rac 12 09 49 650 2 3rt and.





-2-propen-1-one.gif)