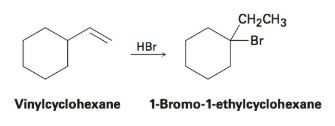
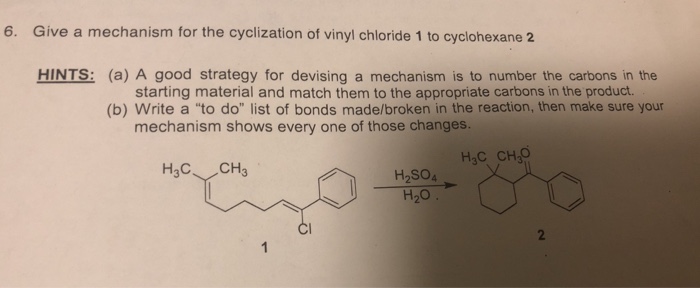
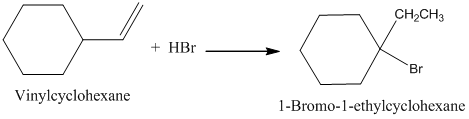
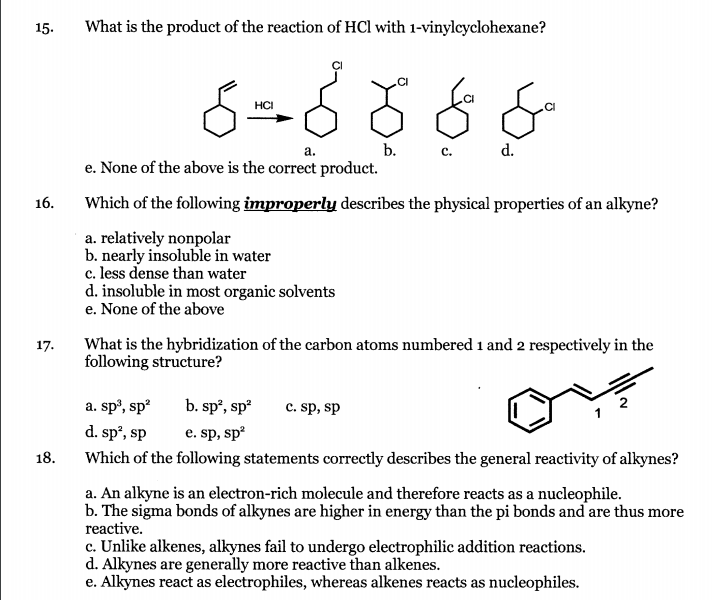
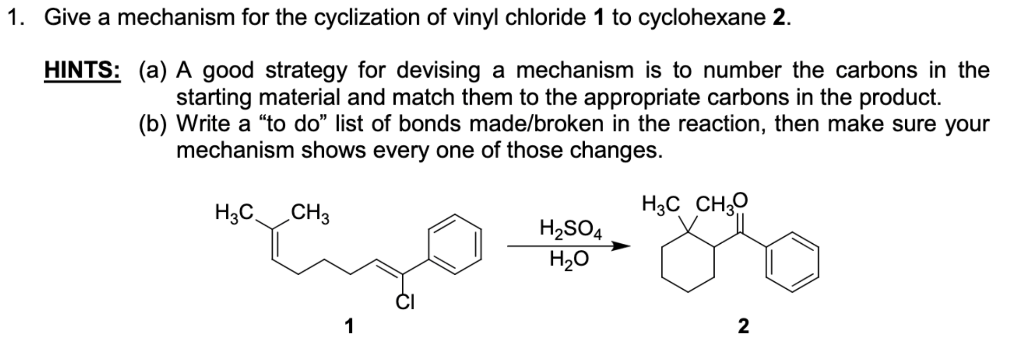
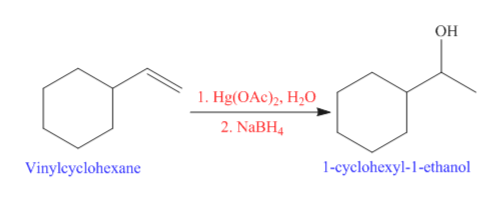
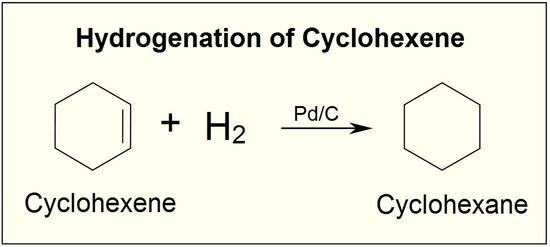
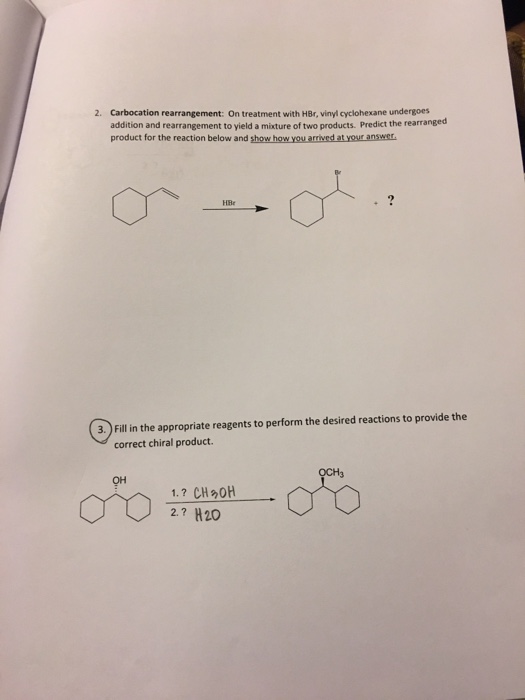
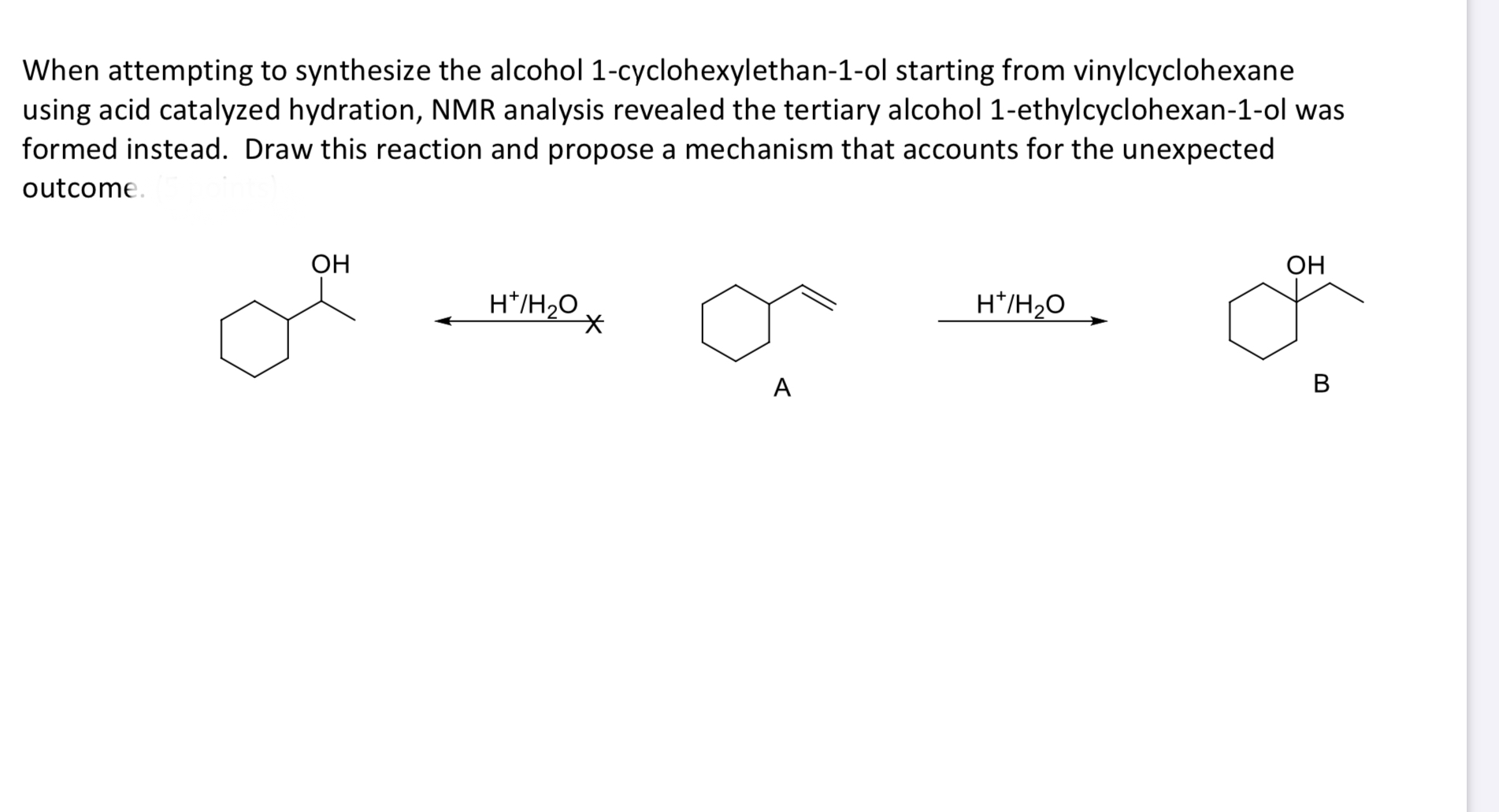
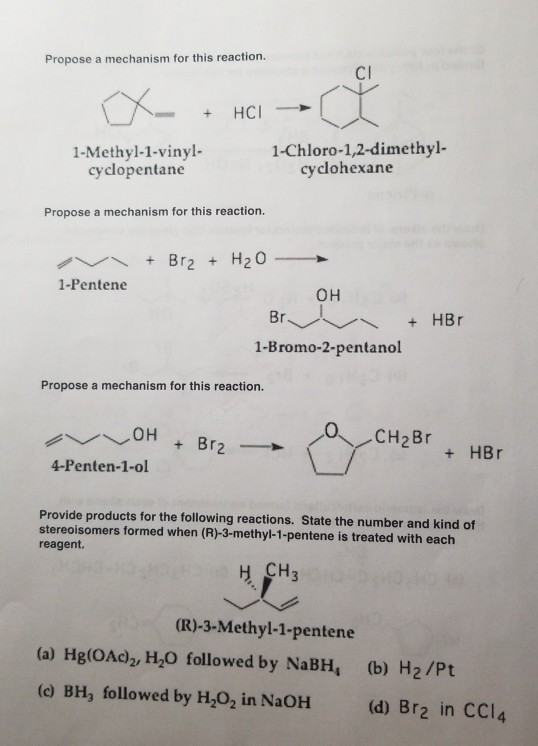
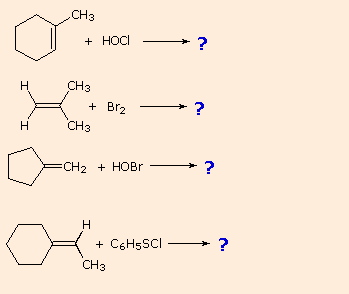
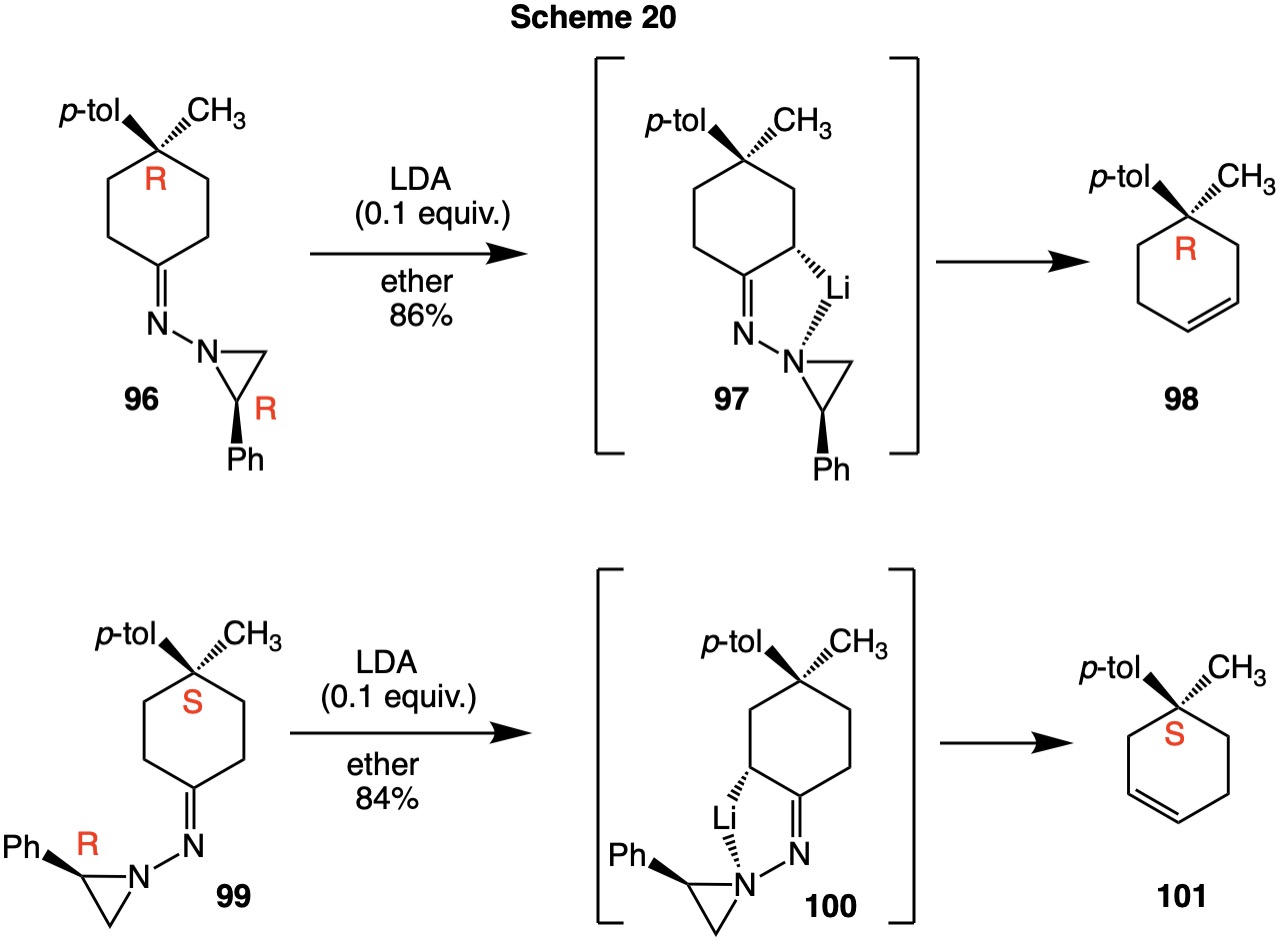
Vinyl Cyclohexane Reactions

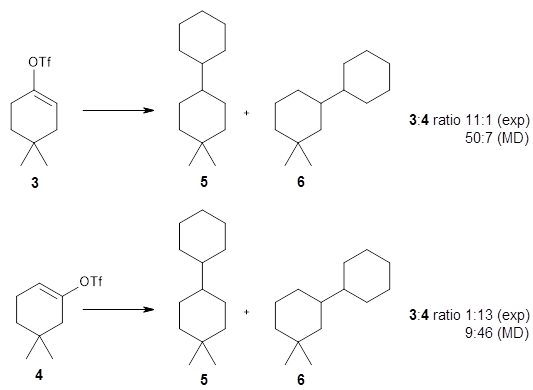
Reaction performed at 0 1 m and 30 c with triisopropylsilane.
Vinyl cyclohexane reactions. Use this link for bookmarking this species for future reference. Use of the information documents and data from the echa website is subject to the terms and conditions of this legal notice and subject to other binding limitations provided for under applicable law the information documents and data made available on the echa website may be reproduced distributed and or used totally or in part for non commercial purposes provided that echa is. 10 17 2016 en english us sds id. Information on this page.
The chemical reaction in this case is a so called ammoximation reaction whereby cyclohexane is reacted with ammonia and hydrogen peroxide at around 90 c in the presence of a titanosilicate catalyst. From wikipedia the free encyclopedia the vinylcyclopropane rearrangement or vinylcyclopropane cyclopentene rearrangement is a ring expansion reaction converting a vinyl substituted cyclopropane ring into a cyclopentene ring. Des réactions secondaires par exemple isomérisation en composés inactifs comportant une double liaison interne et la transformation du monomère en éthyle cyclohexane se produisent pendant la polymérisation du vinyl cyclohexane sur les catalyseurs complexés organométalliques. View chapter purchase book synthetic approaches to small and medium size aza heterocycles in aqueous media.
Enev4520 2 8 p273 avoid release to the environment p303 p361 p353 if on skin or hair. Take off immediately all contaminated clothing. 4 vinyl 1 cyclohexene safety data sheet print date. On a étudié la polymérisation par coordination ionique du vinyl cyclohexane.
1 vinylcyclohexane permanent link for this species. This procedure is one of the key methods to form fused ring systems. The course of the reactions of benzoyl peroxide with cyclohexane and cyclohexene may be accounted for by a mechanism involving free radicals and radical chain reactions.
















.jpg)