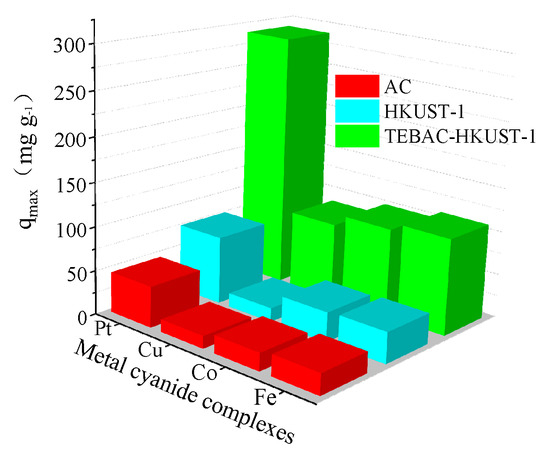
Vinyl Cyanide Synthesis

In terms of its molecular structure it consists of a vinyl group linked to a nitrile it is an important monomer for the manufacture of useful plastics such as polyacrylonitrile.
Vinyl cyanide synthesis. Linear formula ch 2 chcn. It degrades before melting. Ec index number 203 466 5. Beilstein reaxys number 605310.
Pubchem substance id 24846969. Linear formula ch 2 chcn. Polyacrylonitrile pan also known as polyvinyl cyanide and creslan 61 is a synthetic semicrystalline organic polymer resin with the linear formula c 3 h 3 n n though it is thermoplastic it does not melt under normal conditions. It has a pungent odor of garlic or onions.
Vinyl cyanide also prepared by passing the mixture of ammonia steam and air over the catalyst like oxide of the chemical elements molybdenum or cobalt. The synthesis of nitriles is applicable to a broad range of ketones. Product formations are observed in experiments. After an initial.
It melts above 300 c if the heating rates are 50 degrees per minute or above. Acrylonitrile is an organic compound with the formula ch 2 chcn. Molecular weight 53 06. Cyanide catalyst hydrocyanic acid reaction vinyl cyanide prior art date 1944 11 11 legal status the legal status is an assumption and is not a legal conclusion.
Vinyl cyanide cas number 107 13 1. The vinyl cyanide sc2h3cnd or cyano ethylene is an excellent proto type to investigate considering that it is the simplest alkene nitrile and its existence in cold molecular clouds has been confirmed by the observation41 42 of rotational transitions. Beilstein reaxys number 605310. It is a colorless volatile liquid although commercial samples can be yellow due to impurities.
Almost all pan resins are copolymers made from. Vinyl cyanide cas number 107 13 1. Molecular weight 53 06. Stabilised with hydroquinone monomethyl ether for synthesis synonym.
Molecular weight 53 06. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed expired lifetime application number us563098a inventor charles r harris. This research not only provides a new approach for allylic amines derivatives in organic synthesis but also offers valuable mechanistic insights into this novel 1 3 proton shift transfer reaction. Oxazoles function as nitrile equivalents in a cyanide free dual pd cuh catalyzed protocol for the asymmetric markovnikov hydrocyanation of vinyl arenes and the anti markovnikov hydrocyanation of terminal olefins.
For synthesis 99 synonym. Linear formula ch 2 chcn. Pubchem substance id 329828013.